“What is Polarity” Everything You Need to Know: Unveiling the Forces That Shape Our World
Have you ever wondered why magnets always have two distinct ends or why water droplets form on a windowpane? These everyday phenomena are manifestations of a fundamental concept in science: polarity. But what is polarity exactly? In this comprehensive guide, we’ll explore the definition, history, mechanisms, and diverse applications of polarity. Whether you’re a science enthusiast, a student, or simply curious about the forces at play in the natural world, this article will help you understand why polarity matters and how it influences everything from chemical reactions to everyday technology.
Introduction: Discover the Hidden Forces of Polarity
Imagine a world without magnets, without water’s unique behavior, or without the remarkable interactions that allow life to exist as it does. At the heart of many of these phenomena lies polarity—the distribution of electrical charges leading to positive and negative regions. Polarity is not only a cornerstone of chemistry and physics but also a concept that impacts our daily lives in profound ways.
Did you know?
Polarity is what gives water its “sticky” properties, making it essential for life. It also underpins the behavior of molecules, the operation of electronic devices, and even the patterns we see in nature.
In this article, we will cover:
- A clear and straightforward definition of polarity.
- The historical context and milestones that shaped our understanding of polarity.
- An in-depth exploration of the various types of polarity in molecules, magnets, and more.
- Real-world examples and case studies illustrating how polarity is observed and applied.
- The importance of polarity in scientific research, technology, and everyday life.
- Common misconceptions and frequently asked questions about polarity.
- Modern relevance and current trends in the study and application of polarity.
- Practical insights into how understanding polarity can enhance decision-making and spark innovation.
By the end of this post, you’ll have a comprehensive understanding of what is polarity and appreciate its central role in shaping the physical, chemical, and even biological world. Let’s embark on this fascinating journey into the science of polarity.
What is Polarity? A Straightforward Definition
Polarity refers to the presence of distinct and opposite charges or properties within a system. In scientific terms, it generally describes a separation of electrical charge leading to a molecule, magnet, or physical system having distinct positive and negative regions.
Essential Characteristics of Polarity
Charge Separation:
At its core, polarity involves an unequal distribution of electrons in a molecule or material, resulting in regions of partial positive and partial negative charge.Dipole Moment:
A quantitative measure of polarity is the dipole moment, which reflects the strength and direction of the charge separation. The greater the dipole moment, the more polar a molecule is.Molecular Geometry:
The shape of a molecule plays a crucial role in determining its polarity. Even if individual bonds are polar, the overall molecule may be non-polar if the molecular geometry causes the dipole moments to cancel out.Universal Principle:
Polarity isn’t confined to chemistry. It also describes physical systems such as magnets, where north and south poles are distinctly separated, and even social or ideological contexts, where opposing viewpoints or forces are in balance.
Understanding these characteristics provides the foundation for exploring how polarity influences the behavior of molecules, the properties of materials, and a wide range of phenomena in our world.
Historical and Contextual Background
The concept of polarity has evolved over centuries as scientists have unraveled the mysteries of electricity, magnetism, and molecular structure. Let’s explore some key milestones in the history of polarity.
Early Observations and Discoveries
Ancient Magnetism:
The ancient Greeks were among the first to observe natural magnets, known as lodestones, which have distinct north and south poles. This early observation laid the groundwork for understanding magnetic polarity.Electrostatic Phenomena:
In the 17th century, scientists like William Gilbert began studying magnetism and static electricity. Gilbert’s work, published in De Magnete (1600), distinguished between magnetic and electric phenomena, hinting at the concept of polarity.
The 18th and 19th Centuries: Foundations of Modern Science
Coulomb’s Law:
In the 1780s, Charles-Augustin de Coulomb quantified the force between electric charges with what is now known as Coulomb’s Law. This mathematical description of electrical interactions was a significant step toward understanding molecular polarity.Discovery of the Electron:
The discovery of the electron in the late 19th century by J.J. Thomson revolutionized the field of atomic physics. The electron’s negative charge and its behavior in atoms provided critical insight into how polarity arises at the molecular level.Dipole Moments and Molecular Polarity:
In the early 20th century, scientists began to understand that the spatial arrangement of electrons in molecules determines their polarity. Experiments and theoretical models led to the concept of the dipole moment, a measure that quantifies the degree of polarity in a molecule.
Notable Historical Anecdotes
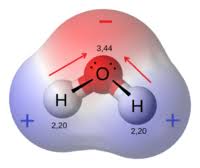
The Tale of the Water Molecule:
One of the most famous examples of molecular polarity is water (H₂O). Early studies of water revealed its unique properties—such as its high surface tension and solvent abilities—are a direct result of its polar nature. The bent shape of the water molecule, with a significant dipole moment, has been pivotal in understanding chemical interactions in biological systems.Magnetic Discoveries in Navigation:
The ancient use of magnetic compasses for navigation also underscores the importance of polarity. Early mariners relied on the consistent orientation of Earth’s magnetic field—a phenomenon of polarity—to traverse vast oceans.
These historical developments illustrate how the study of polarity has deep roots in our scientific heritage and continues to be a dynamic field of inquiry today.
In-Depth Exploration: Understanding the Mechanisms and Types of Polarity
To fully grasp what is polarity, it is essential to delve into its various forms and explore the mechanisms behind it. This section provides a detailed look at different types of polarity and their applications.
1. Molecular Polarity
a. Polar and Non-Polar Molecules
Definition:
A molecule is considered polar if it has an uneven distribution of electrons, resulting in a net dipole moment. Conversely, a molecule is non-polar if its electron distribution is even, causing any individual dipoles to cancel out.Examples:
- Water (H₂O):
Water is a classic example of a polar molecule. Its bent molecular geometry and the difference in electronegativity between oxygen and hydrogen result in a significant dipole moment. - Carbon Dioxide (CO₂):
Despite having polar bonds, CO₂ is a non-polar molecule because its linear shape causes the dipole moments to cancel each other out.
- Water (H₂O):
b. Electronegativity and Bond Polarity
Electronegativity Differences:
The difference in electronegativity between atoms in a bond determines the polarity of that bond. The greater the difference, the more polar the bond.Bond Dipoles:
In a molecule, each polar bond contributes a bond dipole. The overall polarity of the molecule depends on both the magnitude of these dipoles and the molecule’s geometry.
c. Intermolecular Forces and Solubility
Hydrogen Bonding:
Polar molecules, such as water, can form hydrogen bonds—a strong type of intermolecular force. Hydrogen bonding is responsible for many of water’s unique properties, including its high boiling point and surface tension.Solubility:
The polarity of a molecule plays a critical role in its solubility. “Like dissolves like” is a common adage in chemistry, meaning polar substances tend to dissolve in polar solvents, while non-polar substances dissolve in non-polar solvents.
2. Magnetic Polarity
a. Understanding Magnetic Poles
Magnetic Dipoles:
Every magnet has two poles—north and south. These poles are regions where the magnetic field is strongest, and they always occur in pairs.Attraction and Repulsion:
Opposite poles (north and south) attract each other, while like poles repel. This fundamental property of magnets is due to the alignment of magnetic dipoles at the atomic level.
b. Applications in Technology
Compasses and Navigation:
The consistent orientation of Earth’s magnetic field allows compasses to work reliably, making them essential tools for navigation.Electronic Devices:
Magnetic polarity is also crucial in the functioning of many electronic devices, such as hard drives, speakers, and electric motors, where controlled magnetic fields are used to store data and generate motion.
3. Other Forms of Polarity
a. Ionic Polarity
Ionic Bonds:
Ionic compounds consist of positively and negatively charged ions. The polarity in these compounds is due to the complete transfer of electrons from one atom to another, resulting in charged particles that attract each other.Example:
Sodium chloride (NaCl) is an ionic compound where sodium (Na⁺) and chloride (Cl⁻) ions are held together by electrostatic forces.
b. Polarization in Materials
Dielectric Materials:
When an electric field is applied to an insulator (dielectric), the molecules align such that there is a slight separation of charge. This process, known as polarization, is fundamental in the operation of capacitors.Applications:
Polarization is used in various applications, from improving the performance of electronic components to enhancing the properties of optical materials.
4. Real-World Examples and Case Studies
a. Water and Life
Essential Solvent:
Water’s polarity is central to its role as the “universal solvent.” It dissolves a wide range of substances, facilitating chemical reactions essential for life.Case Study:
In biological systems, water’s polar nature enables it to transport nutrients, regulate temperature, and support metabolic processes. For example, the folding of proteins, which is critical for their function, is influenced by the interaction between water and the polar regions of amino acids.
b. Magnetic Storage Devices
Data Storage:
Modern hard drives rely on the principles of magnetic polarity to store data. Tiny magnetic domains are manipulated to represent binary information, enabling the storage and retrieval of vast amounts of data.Case Study:
Advances in magnetic storage have revolutionized the digital age, allowing for the miniaturization of devices and the exponential growth of information technology.
c. Polarity in Everyday Products
Cleaning Agents:
The polar nature of water and other solvents is exploited in cleaning products. Detergents, for example, have both polar (hydrophilic) and non-polar (hydrophobic) ends, enabling them to emulsify and remove grease effectively.Case Study:
The formulation of effective cleaning agents involves a deep understanding of polarity. By designing molecules that can interact with both water and oil, chemists create products that work in a wide range of conditions.
Importance, Applications, and Benefits of Understanding Polarity
Recognizing what is polarity is crucial for various fields, from science and engineering to everyday life. Here’s why a solid grasp of polarity matters:
1. Scientific and Industrial Applications
Chemical Reactions:
Polarity plays a fundamental role in determining how substances interact. Understanding molecular polarity helps chemists predict reaction outcomes, design new materials, and develop pharmaceuticals.Material Science:
The properties of materials—such as conductivity, strength, and flexibility—are often influenced by polarity. Innovations in nanotechnology, electronics, and energy storage depend on manipulating polar interactions at the molecular level.Environmental Science:
Polarity affects the solubility of pollutants in water, influencing how contaminants spread and how they can be removed from the environment. This knowledge is critical for developing effective environmental remediation strategies.
2. Everyday Life and Practical Benefits
Cooking and Nutrition:
The behavior of water in cooking, such as its ability to dissolve flavors and nutrients, is rooted in its polarity. Understanding this helps in developing better culinary techniques and food preservation methods.Household Products:
From cleaning agents to personal care products, many household items are formulated based on principles of polarity. This ensures that products are effective, safe, and environmentally friendly.Health and Medicine:
In medicine, polarity is crucial in drug design and delivery. The solubility and absorption of drugs are often determined by their polar properties, influencing their effectiveness and side effects.
3. Educational and Research Advancements
STEM Education:
Grasping the concept of polarity is a foundational element in science education. It builds a deeper understanding of physical and chemical properties, fostering critical thinking and innovation among students.Research and Innovation:
Ongoing research into the applications of polarity continues to drive advancements in multiple fields. This includes the development of smart materials, improved energy storage systems, and breakthroughs in biotechnology.
4. Broader Cultural and Technological Impact
Interdisciplinary Connections:
Polarity connects diverse fields—from physics and chemistry to biology and engineering. Understanding these connections can inspire cross-disciplinary collaborations and novel technological solutions.Global Relevance:
The principles of polarity are universal, affecting both natural systems and human-made technologies worldwide. This universality makes it a key concept in global scientific and technological discussions.
Addressing Common Misconceptions and FAQs about Polarity
Despite its fundamental role in science and everyday life, several misconceptions about polarity persist. Let’s clarify some common myths and provide clear answers to frequently asked questions.
Common Misconceptions
Misconception 1: Polarity Only Applies to Molecules
Reality:
While molecular polarity is a major focus, the concept of polarity also applies to magnets, ionic compounds, and even social or political contexts where opposing forces or ideas are at play.Misconception 2: Only Polar Molecules Are Important in Chemistry
Reality:
Both polar and non-polar molecules are essential in chemistry. Non-polar molecules, though lacking a dipole moment, play critical roles in processes such as energy storage, lubrication, and biological membrane formation.Misconception 3: Polarity Is a Static Property
Reality:
Polarity can change under different conditions. For example, the polarity of a molecule can be influenced by its environment, such as changes in temperature or the presence of an electric field.
Frequently Asked Questions (FAQs)
Q: What exactly is polarity?
A:
Polarity is the separation of electrical charge within a molecule, material, or system, leading to distinct positive and negative regions.Q: How do you measure polarity in a molecule?
A:
The dipole moment is used to measure a molecule’s polarity. It quantifies the strength and direction of the charge separation, typically expressed in Debye units.Q: Why is water considered a polar molecule?
A:
Water has a bent molecular structure and a significant electronegativity difference between oxygen and hydrogen, resulting in a net dipole moment with a partial negative charge near the oxygen and partial positive charges near the hydrogens.Q: Can non-polar substances exhibit any polarity?
A:
Under certain conditions, non-polar substances can exhibit induced polarity when exposed to external electric fields or when interacting with polar molecules.Q: How does polarity affect the behavior of materials?
A:
Polarity influences solubility, boiling and melting points, and intermolecular forces, which in turn affect the physical and chemical properties of materials.
Modern Relevance and Current Trends in the Study of Polarity
In today’s rapidly evolving world, the study and application of what is polarity continue to expand. Here are some of the modern trends and developments shaping this field:
1. Advances in Material Science and Nanotechnology
Smart Materials:
Researchers are developing materials that can change their properties in response to external stimuli by exploiting polarity. These smart materials have applications in flexible electronics, adaptive optics, and responsive surfaces.Nanotechnology:
At the nanoscale, controlling polarity allows scientists to design materials with unique electronic, optical, and mechanical properties. This has led to breakthroughs in fields like quantum computing and targeted drug delivery.
2. Renewable Energy and Environmental Applications
Solar Cells:
The efficiency of photovoltaic cells depends significantly on the polarity of the materials used. Advances in controlling molecular polarity have contributed to the development of more efficient and durable solar panels.Pollution Control:
Understanding how pollutants interact with water (a polar solvent) helps in designing effective methods for environmental remediation. Research into polarity is essential for improving water purification and waste treatment technologies.
3. Technological Innovations in Electronics
Semiconductors and Transistors:
Modern electronics rely on materials with well-defined polar properties. The development of advanced semiconductors, which are essential for transistors and microchips, depends on manipulating the polarity at the atomic level.Display Technologies:
Liquid crystals, used in displays, exhibit unique polar properties that allow them to modulate light. Innovations in controlling these properties have led to improvements in screen resolution and energy efficiency.
4. Educational Initiatives and Research Collaborations
Interactive Learning Tools:
Advanced simulation software and virtual labs now allow students to explore polarity and its effects in a dynamic, interactive environment. This hands-on approach fosters a deeper understanding of complex scientific principles.Interdisciplinary Research:
Collaborations between chemists, physicists, biologists, and engineers are driving innovations that span multiple fields. Understanding polarity is central to these interdisciplinary efforts, leading to novel applications and breakthroughs.
Conclusion: Embracing the Power of Polarity
Our extensive exploration of what is polarity has taken us through definitions, historical milestones, mechanisms, real-world applications, and modern trends. Here are the key takeaways:
Definition and Fundamentals:
Polarity is the separation of electrical charges within a molecule, material, or system, resulting in distinct positive and negative regions. It is quantified by the dipole moment and is influenced by molecular geometry and electronegativity.Historical Context:
From the early observations of magnetism and static electricity to modern advancements in material science, our understanding of polarity has evolved dramatically, shaping countless scientific and technological innovations.Mechanisms and Applications:
Whether it’s the polar nature of water that sustains life, the behavior of magnets in technology, or the design of smart materials in nanotechnology, polarity is a fundamental concept that underpins a wide range of phenomena.Modern Relevance:
The study of polarity remains crucial in today’s world, driving advancements in renewable energy, electronics, environmental science, and interdisciplinary research.
Call to Action
Now that you have a comprehensive understanding of what is polarity, we encourage you to:
- Reflect: Consider how polarity affects the world around you—from the behavior of water and magnets to the functioning of your electronic devices.
- Explore Further: Delve deeper into related topics such as molecular chemistry, electromagnetism, and materials science to broaden your scientific knowledge.
- Engage: Share your thoughts, questions, or experiences in the comments below. How has learning about polarity influenced your understanding of everyday phenomena?
- Share: If you found this article informative and engaging, please share it with friends, educators, or anyone interested in the fascinating world of science.
By embracing the concept of polarity, we not only enhance our understanding of the natural world but also empower ourselves to innovate and solve practical problems. Polarity is more than just a scientific term—it’s a window into the forces that shape our universe.
Additional Resources and References
For those eager to further explore what is polarity, here are some reputable sources and further reading materials:
Books and Academic Texts:
- “Physical Chemistry” by Peter Atkins and Julio de Paula – An in-depth look at the principles of molecular polarity and intermolecular forces.
- “Introduction to Electrodynamics” by David J. Griffiths – A comprehensive guide to the principles of electricity and magnetism.
- “Molecular Quantum Mechanics” by Peter Atkins and Ronald Friedman – Explores how quantum mechanics explains the behavior of polar molecules.
Online Educational Resources:
- Khan Academy – Chemistry – Videos and articles explaining molecular polarity, dipole moments, and related concepts.
- MIT OpenCourseWare – Free course materials on electromagnetism and physical chemistry.
- HyperPhysics – An online resource with detailed explanations of polarity, electromagnetism, and other related topics.
Research Journals and Articles:
- Journal of Physical Chemistry – Articles on molecular polarity and its applications in chemical research.
- Nature Materials – Research on advanced materials where polarity plays a key role.
Workshops and Online Courses:
- Platforms such as Coursera, edX, and Udemy offer courses in physical chemistry, electromagnetism, and materials science that delve into the concept of polarity.
Final Thoughts
Polarity is a powerful and pervasive concept that governs the behavior of molecules, materials, and even technological systems. By understanding what is polarity, we gain valuable insights into the forces that shape our natural and engineered worlds—from the simple phenomenon of water’s surface tension to the sophisticated design of electronic devices. As you continue your exploration of science, remember that even the tiniest charge separations can have monumental impacts.
Thank you for joining us on this in-depth journey into the world of polarity. We hope this article has enriched your understanding and sparked your curiosity to explore further. If you enjoyed this post, please share it, leave your feedback or questions in the comments below, and help spread the wonder of scientific discovery.