What Is pH? Everything You Need to Know
Have you ever wondered why a lemon tastes sour, or why soap feels slippery? What is pH, and how does this fundamental concept affect everything from the water we drink to the health of our ecosystems? In this comprehensive guide, we will explore what is pH—its definition, key characteristics, historical evolution, practical applications, and modern relevance. Whether you’re a student, educator, scientist, or simply a curious reader, this post will equip you with all the knowledge you need to understand pH and its importance in everyday life.
Introduction: The Hidden Measure That Shapes Our World
Imagine a world where the quality of your drinking water, the efficiency of your cleaning products, and even the proper functioning of your body depend on one crucial scale. The pH scale—a simple numerical system ranging from 0 to 14—is at the heart of these phenomena. Did you know that maintaining the correct pH balance in your body is critical for survival, and that even a small shift can lead to serious health problems? Similarly, industries rely on pH control for manufacturing everything from pharmaceuticals to food products.
In this article, we will cover:
- A clear and straightforward definition of pH and its fundamental properties.
- The essential characteristics that define pH, including its measurement and role in classifying substances as acidic, neutral, or alkaline.
- A historical and contextual background detailing the origins of the pH scale and key milestones in its evolution.
- An in-depth exploration of the science behind pH, including its applications in biology, environmental science, industry, and everyday life.
- Real-world examples and case studies illustrating how pH is observed and applied—from water treatment plants to soil science and human physiology.
- The importance, applications, and benefits of understanding pH in fields such as health, agriculture, manufacturing, and environmental conservation.
- Common misconceptions and FAQs that clarify misunderstandings about pH and its measurement.
- Modern relevance and current trends showing how advancements in technology and research continue to enhance our understanding of pH.
- A conclusion that summarizes the key points and provides a call-to-action for further learning and engagement.
By the end of this guide, you’ll have a comprehensive understanding of what is pH, why it is crucial for various aspects of life and industry, and how this simple scale influences our world. Let’s dive into the fascinating world of pH!
Section 1: Defining pH
What Is pH?
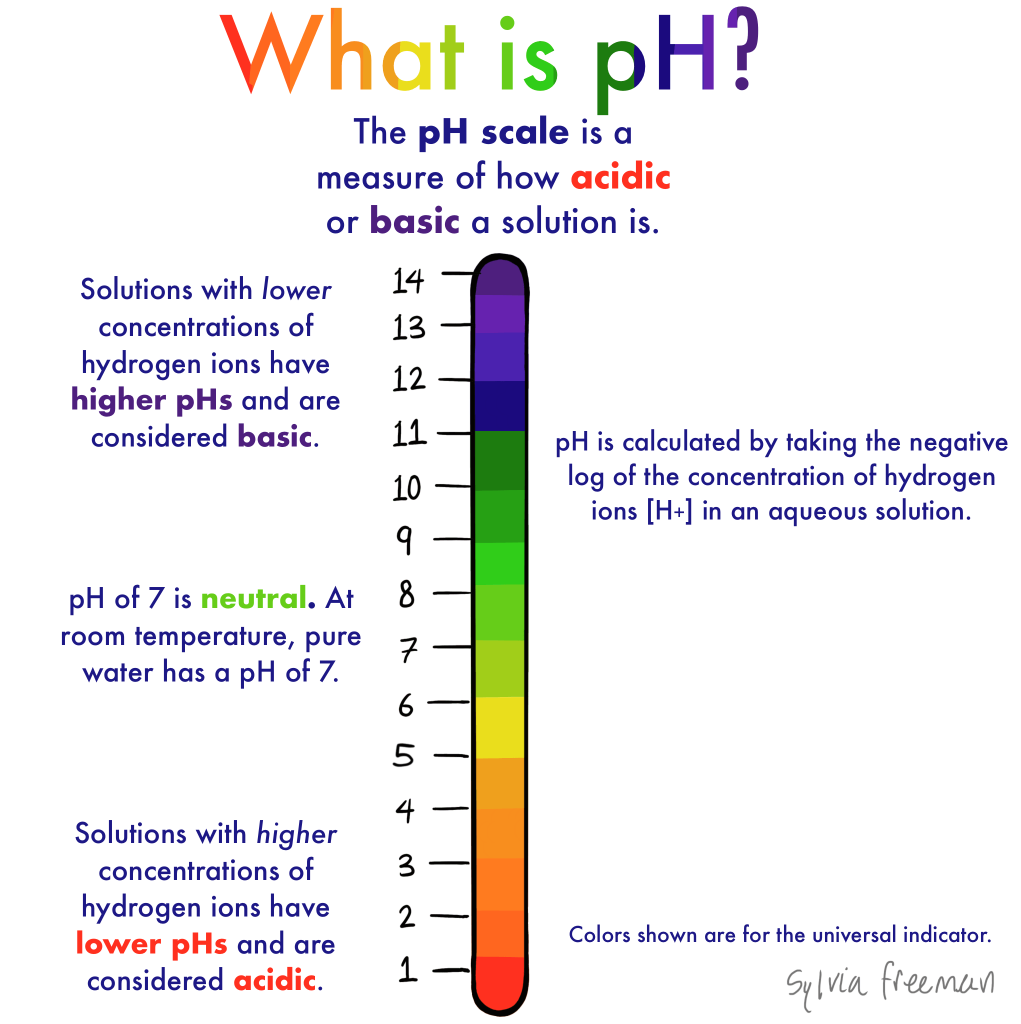
At its most basic level, pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm (base 10) of the concentration of hydrogen ions (H⁺) in a solution. The pH scale ranges from 0 to 14:
- Acidic solutions have a pH less than 7.
- Neutral solutions have a pH of exactly 7.
- Alkaline (or basic) solutions have a pH greater than 7.
Straightforward Definition:
pH is a numerical scale used to specify the acidity or alkalinity of a solution, based on the concentration of hydrogen ions present. A lower pH value indicates a higher concentration of hydrogen ions (acidic), while a higher pH value indicates a higher concentration of hydroxide ions (alkaline).
Essential Characteristics of pH
When we ask what is pH, several important properties define this concept:
Logarithmic Scale:
The pH scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration. For example, a solution with a pH of 4 is ten times more acidic than one with a pH of 5.Indicator of Chemical Nature:
pH is a critical indicator that determines whether a substance is acidic, neutral, or alkaline. This classification affects how substances interact in chemical reactions, biological processes, and industrial applications.Biological Importance:
Many biological systems require a specific pH range to function optimally. For example, human blood is maintained at a slightly alkaline pH of around 7.4, and even small deviations can have serious health implications.Environmental Relevance:
pH affects water quality, soil fertility, and ecosystem health. The balance of pH in natural waters and soils is essential for the survival of aquatic life and the productivity of agriculture.Industrial Applications:
In industry, controlling pH is vital for processes such as fermentation, chemical manufacturing, and wastewater treatment. Accurate pH measurements ensure product quality and environmental compliance.
Understanding these characteristics helps us appreciate why pH is a fundamental concept in both science and everyday life.
Section 2: Historical and Contextual Background
The Origins of the pH Scale
The concept of pH was developed in the early 20th century as scientists sought a way to measure the acidity or alkalinity of solutions quantitatively. The breakthrough came in 1909 with the work of Danish chemist Søren Peter Lauritz Sørensen, who introduced the pH scale.
Key Milestones in the Evolution of pH
Sørensen’s Contribution (1909):
Søren Sørensen proposed the pH scale as a simple and effective means of expressing the acidity or alkalinity of a solution. By taking the negative logarithm of the hydrogen ion concentration, he created a scale that could easily distinguish between strongly acidic and strongly alkaline solutions.Adoption in Chemistry and Biology:
Following its introduction, the pH scale quickly became a standard tool in chemistry and biology. It allowed scientists to conduct experiments with greater precision and consistency, particularly in fields such as biochemistry, where pH plays a crucial role in enzyme activity and metabolic processes.Technological Advances:
With the development of more sophisticated pH meters and indicators, the measurement of pH became more accurate and accessible. These technological advancements have enabled a wide range of applications in research, industry, and environmental monitoring.
Historical Anecdotes and Context
The Early Laboratory:
Early chemists relied on natural indicators like litmus paper to determine the pH of a solution. The invention of the pH meter revolutionized the process, allowing for precise and immediate measurements that transformed laboratory practices.Industrial Revolution Impact:
During the Industrial Revolution, the need for precise chemical measurements grew rapidly. Industries such as paper manufacturing, textile production, and food processing began to adopt pH measurements to improve product quality and safety.Environmental Milestones:
The pH scale also played a crucial role in understanding environmental issues such as acid rain. Scientists used pH measurements to track the acidification of lakes and rivers, leading to regulatory policies aimed at reducing air pollution and its harmful effects on ecosystems.
For further historical context, consider reading about the development of the pH scale on reputable sources such as the Encyclopedia Britannica and academic publications on the history of chemistry.
Section 3: In-Depth Exploration of pH
To fully understand what is pH, we need to explore its scientific basis, how it is measured, and its diverse applications in various fields.
The Science Behind pH
Understanding the pH Scale
Definition Recap:
pH is defined as the negative logarithm of the hydrogen ion concentration in a solution:This equation shows that as the concentration of hydrogen ions increases, the pH decreases, and vice versa.
Logarithmic Nature:
The logarithmic scale means that small changes in pH represent significant changes in hydrogen ion concentration. For example, a change from pH 3 to pH 4 represents a tenfold decrease in acidity.
Acidic, Neutral, and Alkaline Solutions
Acidic Solutions:
Solutions with a pH less than 7 are acidic. They have a higher concentration of hydrogen ions. Common examples include lemon juice, vinegar, and stomach acid.Neutral Solutions:
A neutral solution has a pH of exactly 7. Pure water is the most familiar example.Alkaline Solutions:
Solutions with a pH greater than 7 are alkaline (or basic). They contain more hydroxide ions than hydrogen ions. Examples include baking soda solutions, soapy water, and bleach.
Methods of Measuring pH
Traditional Methods
Litmus Paper:
One of the earliest methods for measuring pH, litmus paper changes color when dipped in a solution. Red litmus turns blue in alkaline solutions, and blue litmus turns red in acidic solutions.pH Indicators:
Various chemical indicators have been developed that change color at specific pH ranges. These are useful in laboratory settings and educational demonstrations.
Modern Techniques
pH Meters:
Electronic pH meters provide a digital reading of a solution’s pH. They are widely used in scientific research, industrial processes, and environmental monitoring for their precision and ease of use.Automated Systems:
In large-scale industrial and environmental applications, automated pH monitoring systems are employed. These systems continuously measure and adjust the pH in processes such as water treatment and fermentation.
Real-World Applications of pH
Environmental Applications
Water Quality Monitoring:
pH is a critical parameter in assessing water quality. Both natural and polluted water bodies have their pH levels monitored to ensure they are safe for human consumption and aquatic life. Acid rain, for instance, can lower the pH of lakes and streams, affecting biodiversity.Soil Health:
Soil pH influences nutrient availability and plant growth. Farmers and agronomists regularly test soil pH to determine the need for lime (to raise pH) or sulfur (to lower pH), ensuring optimal crop yields.
Biological and Medical Applications
Human Physiology:
The human body requires a tightly regulated pH level, especially in the blood (around 7.4). Deviations from this range can lead to severe health issues. Medical professionals use pH measurements to monitor and treat conditions such as acidosis or alkalosis.Biochemical Processes:
Enzymatic reactions and metabolic pathways in living organisms are highly dependent on pH. Researchers study pH to understand how cells function and to develop treatments for various diseases.
Industrial Applications
Chemical Manufacturing:
Many industrial processes, such as the production of paper, textiles, and detergents, rely on precise pH control to ensure product quality and process efficiency.Food and Beverage Industry:
The flavor, safety, and preservation of food products are influenced by pH levels. For example, the fermentation process in brewing and winemaking requires careful pH monitoring to produce high-quality products.Pharmaceutical Production:
In the pharmaceutical industry, pH control is critical for ensuring the stability and efficacy of medications. Many drugs are formulated to work optimally within specific pH ranges.
Agricultural Applications
- Crop Production:
As mentioned earlier, soil pH plays a vital role in agriculture. Farmers adjust the pH of their fields to enhance nutrient uptake by crops, leading to improved productivity and sustainability.
Case Studies and Real-World Examples
Case Study 1: Acid Rain and Environmental Impact
Overview:
Acid rain, caused by industrial emissions of sulfur dioxide and nitrogen oxides, can significantly lower the pH of rainwater. This phenomenon has severe impacts on lakes, forests, and human-made structures.Impact:
Low pH levels in water bodies can harm aquatic life, corrode infrastructure, and lead to the leaching of toxic metals into the soil.Key Takeaway:
Monitoring and managing pH is essential for mitigating the harmful effects of acid rain and protecting ecosystems.
Case Study 2: pH in Water Treatment Plants
Overview:
Water treatment plants rely on precise pH control to remove contaminants and ensure that water is safe for consumption. The treatment process involves adding chemicals to adjust the pH, which helps in coagulating and removing impurities.Impact:
Consistent pH monitoring and adjustment ensure that water treatment processes are efficient, reducing the risk of waterborne diseases.Key Takeaway:
pH is a critical parameter in public health and environmental management, highlighting its importance in infrastructure and community well-being.
Case Study 3: pH and Human Health
Overview:
The pH of bodily fluids, such as blood and gastric acid, is crucial for proper physiological function. Deviations from the normal pH range can indicate or lead to health problems.Impact:
For example, an imbalance in blood pH (acidosis or alkalosis) can be life-threatening and requires immediate medical attention.Key Takeaway:
Understanding pH is vital for medical diagnostics and treatment, emphasizing its role in maintaining human health.
For more detailed case studies, reputable sources include the World Health Organization (WHO) and the Environmental Protection Agency (EPA).
Section 4: Importance, Applications, and Benefits of Understanding pH
Why Understanding pH Matters
Understanding what is pH is essential because it impacts virtually every aspect of our lives. Here’s why this concept is so important:
Public Health and Safety
Medical Diagnostics:
Accurate pH measurements are crucial for diagnosing and treating health conditions. Maintaining proper pH levels in bodily fluids ensures that metabolic processes function correctly.Water Quality:
pH is a fundamental parameter in water quality testing. Ensuring that water is within a safe pH range is essential for preventing disease and promoting public health.
Environmental Protection
Ecosystem Health:
The pH of soil and water directly affects plant growth, aquatic life, and overall ecosystem balance. Sustainable pH management is key to preserving biodiversity and natural resources.Pollution Control:
pH plays a significant role in the treatment of industrial wastewater and the mitigation of environmental pollution. Maintaining a balanced pH helps reduce the harmful effects of contaminants.
Industrial and Economic Benefits
Optimized Manufacturing Processes:
Many industries depend on precise pH control to ensure the quality and efficiency of production. This is particularly true in sectors such as food processing, pharmaceuticals, and chemical manufacturing.Economic Efficiency:
Efficient pH management can reduce waste, lower costs, and improve product quality, contributing to economic growth and sustainability.
Educational and Scientific Advancements
Scientific Literacy:
A solid understanding of pH is a cornerstone of science education. It is essential for students studying chemistry, biology, environmental science, and related fields.Innovative Research:
Advances in pH measurement and control have spurred innovations in various scientific and technological fields, from nanotechnology to biotechnology.
Applications Across Various Domains
In Education
Classroom Learning:
Teaching pH in schools and universities helps students understand chemical reactions, biological processes, and environmental science. Hands-on experiments, such as testing the pH of household substances, make learning interactive and engaging.Research Projects:
Many academic research projects rely on accurate pH measurements to explore topics ranging from cellular biology to climate change.
In Business and Industry
Quality Control:
Industries use pH measurements to maintain quality standards in products. For example, the food and beverage industry monitors pH to ensure product safety and consistency.Product Development:
Companies in sectors such as cleaning products, cosmetics, and pharmaceuticals use pH as a critical factor in product formulation. Understanding pH helps in developing products that are safe, effective, and appealing to consumers.
In Environmental Management
Water and Soil Conservation:
pH is a key indicator of environmental health. Managing the pH of water bodies and soil is crucial for agriculture, forestry, and wildlife conservation.Pollution Mitigation:
Effective pH control in wastewater treatment helps protect natural water resources and prevents environmental degradation.
In Healthcare
Medical Treatments:
pH plays a vital role in various medical treatments, from maintaining the acidity of the stomach for proper digestion to balancing the pH of intravenous fluids.Patient Monitoring:
Regular pH measurements are an important aspect of patient care, particularly in critical care settings, to ensure that bodily functions remain within safe ranges.
For additional insights into the benefits and applications of pH, resources like the American Chemical Society and National Institutes of Health (NIH) offer valuable research and data.
Section 5: Common Misconceptions and FAQs About pH
Debunking Common Misconceptions
Despite its importance, several misconceptions about what is pH persist. Let’s address some of the most common myths:
Misconception #1: pH Only Matters in a Laboratory.
Reality:
pH is a critical factor in everyday life, from the water we drink to the food we eat, and even in our own bodies. It has practical applications in health, industry, and the environment.Misconception #2: The pH Scale Is Linear.
Reality:
The pH scale is logarithmic, meaning that each whole number change represents a tenfold difference in hydrogen ion concentration. This non-linear nature is essential for understanding the magnitude of pH differences.Misconception #3: A Neutral pH of 7 Is the Same for All Substances.
Reality:
While pure water has a neutral pH of 7, the pH of other substances can vary widely based on their chemical composition and environmental conditions.Misconception #4: pH Is Irrelevant Outside of Chemistry.
Reality:
pH plays a vital role in biology, medicine, environmental science, and even economics. It is a universal measure that impacts a wide range of fields.
Frequently Asked Questions (FAQs)
Q: What is pH?
A: pH is a measure of the acidity or alkalinity of a solution, defined as the negative logarithm of the hydrogen ion concentration. A pH below 7 is acidic, a pH of 7 is neutral, and a pH above 7 is alkaline.Q: How is pH measured?
A: pH can be measured using various methods, including litmus paper, pH indicators, and electronic pH meters. Modern pH meters provide precise digital readings and are widely used in both laboratories and industrial settings.Q: Why is pH important for health?
A: Maintaining the correct pH in the human body is essential for proper physiological functioning. For example, human blood is slightly alkaline, with a pH around 7.4, and deviations from this range can lead to serious health issues.Q: How does pH affect the environment?
A: pH levels in water and soil influence the health of ecosystems. For instance, a low pH in water bodies (acidic conditions) can harm aquatic life, while soil pH affects nutrient availability and plant growth.Q: What are some everyday examples of pH?
A: Common examples include the acidity of lemon juice (low pH), the neutrality of pure water (pH 7), and the alkalinity of baking soda (pH around 8.3).
Section 6: Modern Relevance and Current Trends in pH
pH in the Digital and Global Era
In today’s fast-paced world, the concept of what is pH continues to be relevant, not only in science but also in various technological and societal applications.
Technological Innovations
Advanced pH Meters and Sensors:
Technological advancements have led to the development of highly accurate pH meters and sensors that can provide real-time data. These innovations are critical in environmental monitoring, industrial processes, and medical diagnostics.Automated Systems:
Automated pH monitoring systems are now standard in water treatment plants, food processing, and pharmaceutical manufacturing. These systems ensure that processes remain within optimal pH ranges, enhancing product quality and safety.
Environmental and Agricultural Trends
Sustainable Water Management:
With increasing concerns about water quality and availability, monitoring pH levels in natural and treated water is more important than ever. Efforts to combat acid rain, manage industrial discharges, and preserve aquatic ecosystems rely on precise pH measurements.Soil Health and Sustainable Agriculture:
Farmers and agronomists use pH measurements to assess soil health and determine the need for amendments. Sustainable agriculture practices increasingly emphasize maintaining optimal pH levels to enhance crop yields and reduce environmental impact.
Health and Nutritional Developments
The Alkaline Diet Debate:
The popularity of the alkaline diet has sparked debates among nutritionists and medical professionals. While the scientific community continues to research the potential benefits of an alkaline diet, many health enthusiasts believe that balancing the body’s pH can improve overall health.Biomedical Applications:
In healthcare, maintaining proper pH levels is crucial for patient care. Advances in medical technology have improved our ability to monitor and adjust pH in clinical settings, leading to better treatment outcomes.
Policy and Global Initiatives
Environmental Regulations:
Governments worldwide are establishing regulations to monitor and control pH levels in water bodies, industrial discharges, and agricultural runoff. These policies are essential for protecting ecosystems and ensuring public health.International Cooperation:
Global organizations, such as the United Nations and the World Health Organization, continue to emphasize the importance of pH in environmental and public health initiatives, underscoring its relevance in international policy and sustainability efforts.
For further insights into modern trends in pH research and applications, consider exploring resources such as the EPA’s Water Quality Program and WHO’s Water, Sanitation, and Health.
Section 7: Practical Applications and Benefits of Understanding pH
Everyday Benefits
Understanding what is pH empowers individuals to make informed decisions in various aspects of life:
Health and Wellness:
Awareness of pH is crucial for maintaining personal health, from understanding the importance of a balanced diet to ensuring that the products you use (like skincare items) are pH-balanced for your body.Environmental Stewardship:
By understanding pH, you can better appreciate environmental issues such as water pollution and soil degradation, leading to more responsible behaviors and support for sustainable practices.Consumer Empowerment:
Knowledge of pH enables consumers to choose products that are safer and more effective, whether it’s selecting cleaning agents or evaluating the quality of drinking water.
Applications Across Various Domains
In Education
Interactive Science Lessons:
Teachers use pH experiments to demonstrate chemical reactions and environmental processes. Activities like testing the pH of common household substances or examining the effects of acid rain make learning interactive and memorable.Curriculum Development:
Incorporating pH-related topics into science curricula helps students understand fundamental chemical principles and their real-world applications.
In Business and Industry
Quality Control in Manufacturing:
Industries such as food processing, pharmaceuticals, and chemical manufacturing rely on precise pH control to ensure product quality and safety. Understanding pH is essential for optimizing production processes and reducing waste.Innovative Product Development:
Companies that design eco-friendly and pH-balanced products—ranging from personal care items to cleaning agents—benefit from consumer trust and market differentiation.
In Environmental Management
Water Treatment:
pH monitoring is a cornerstone of water treatment processes. By maintaining the appropriate pH levels, treatment plants can effectively remove contaminants and ensure safe drinking water.Soil and Agricultural Management:
Farmers use pH measurements to optimize soil conditions for crop growth. Adjusting soil pH through amendments can improve nutrient availability and enhance agricultural productivity.
In Healthcare
Medical Diagnostics:
Healthcare providers monitor pH levels in blood, urine, and other bodily fluids to diagnose and treat various conditions. Maintaining a balanced pH is critical for metabolic processes and overall health.Therapeutic Interventions:
Treatments that involve pH regulation, such as intravenous fluids and certain medications, are designed based on the understanding of pH’s role in human physiology.
For additional practical applications, websites such as Khan Academy and The Chemistry of pH on Chemguide offer interactive lessons and detailed explanations.
Section 8: Conclusion and Call-to-Action
Summarizing the Essentials
So, what is pH? It is a measure of the acidity or alkalinity of a solution, defined by the concentration of hydrogen ions present. The pH scale, ranging from 0 to 14, categorizes substances as acidic, neutral, or alkaline. Throughout this guide, we have explored:
- A clear definition of pH and its fundamental properties.
- The essential characteristics of pH, including its logarithmic scale, role in chemical reactions, and significance in biological and environmental systems.
- The historical evolution of pH, from its early discovery and the work of Søren Sørensen to modern technological advancements.
- An in-depth exploration of the science behind pH, including its applications in health, industry, agriculture, and environmental management.
- Real-world examples and case studies that illustrate how pH is measured and applied in various contexts, such as water treatment, soil management, and medical diagnostics.
- The importance, applications, and benefits of understanding pH for informed decision-making in everyday life and professional settings.
- Common misconceptions and FAQs that clarify what pH is and dispel myths about its significance.
- Modern relevance and current trends that highlight the role of pH in today’s digital, industrial, and global landscape.
The Importance of Understanding pH
Understanding what is pH is essential because it underpins many aspects of our world—from the proper functioning of our bodies to the health of our environment and the efficiency of industrial processes. Knowledge of pH enables us to:
- Make informed decisions about health, safety, and environmental sustainability.
- Improve product quality and industrial processes through precise control of chemical reactions.
- Enhance scientific literacy and critical thinking skills by understanding the fundamental principles of chemistry.
- Promote sustainable practices and environmental stewardship by managing natural resources responsibly.
Call-to-Action
Now that you have a comprehensive understanding of pH, here are some steps you can take to further engage with this important topic:
Educate Yourself Further:
Explore additional resources on pH and acid-base chemistry. Reputable websites like Khan Academy and Chemguide offer interactive tutorials and detailed explanations.Experiment at Home:
Try simple experiments, such as testing the pH of various household liquids using pH strips. These hands-on activities can deepen your understanding of how pH works in everyday substances.Apply Your Knowledge:
Use your understanding of pH to make informed choices—whether it’s selecting eco-friendly cleaning products, ensuring your water quality is safe, or optimizing your garden’s soil for better plant growth.Share This Guide:
If you found this post helpful, share it with friends, colleagues, or on social media. Spreading awareness about the importance of pH can help others appreciate its role in our lives.Join the Conversation:
We’d love to hear your thoughts and experiences regarding pH. Leave a comment below with your questions, insights, or stories about how pH has impacted your work or daily life.Subscribe for More:
Stay updated on the latest research, technological advancements, and practical applications in chemistry, environmental science, and health by subscribing to our newsletter. Join our community of lifelong learners and informed citizens!
By taking these steps, you contribute to a better understanding of pH and its critical role in shaping our health, environment, and technological progress.
Final Thoughts
pH is far more than just a number on a scale—it is a fundamental concept that affects virtually every aspect of our world. From ensuring the safety of our drinking water and the efficiency of industrial processes to maintaining the delicate balance of our body’s chemistry, pH is a crucial parameter that we rely on daily. Understanding what is pH not only enhances our scientific literacy but also empowers us to make better decisions for our health, our environment, and our future.
Thank you for taking the time to explore this comprehensive guide on pH. We hope it has provided you with valuable insights and practical knowledge that will inspire you to further explore the fascinating world of chemistry and its applications in everyday life.
For further reading and additional resources, please visit:
- Khan Academy – Chemistry
- EPA – Water Quality and pH
- World Health Organization – Water, Sanitation, and Health (WASH)
If you found this guide helpful, please share it on social media, leave your feedback in the comments below, and subscribe to our newsletter for more in-depth articles on science, health, and environmental sustainability.
Let’s harness the power of knowledge to make informed choices and build a healthier, more sustainable world—one accurate pH measurement at a time.
Happy learning, and stay curious!