“What Is Isotopes: Everything You Need to Know”
Isotopes might sound like a complex scientific term reserved for chemistry labs and nuclear reactors, but they are all around us—and they play a crucial role in everything from medical diagnostics to environmental studies. So, what is isotopes? In this comprehensive guide, we will demystify the concept of isotopes, explore their fascinating history and evolution, and discuss their importance and applications in our everyday lives. Whether you’re a student, a science enthusiast, or simply curious about the building blocks of matter, this post will equip you with all the insights you need to understand isotopes.
Introduction
Imagine being able to trace the movement of water through an ecosystem or diagnose a life-threatening disease using a tiny particle. It might sound like science fiction, but it’s made possible by one simple concept: isotopes. Did you know that isotopes of elements have been used to unlock secrets about the age of our planet and even to treat cancer? These incredible variants of atoms provide us with powerful tools to explore and improve our world.
In this article, we will cover:
- A clear and concise definition of isotopes.
- The key properties and characteristics that define isotopes.
- The historical evolution and milestones in the understanding of isotopes.
- An in-depth exploration of different types of isotopes with real-world examples and case studies.
- The significance, applications, and benefits of isotopes across science, medicine, environmental studies, and industry.
- Common misconceptions and frequently asked questions about isotopes.
- Modern trends and current research shaping the future of isotope applications.
By the end of this guide, you’ll have a deep understanding of what is isotopes, why they are important, and how they continue to impact various fields in our modern world.
What Is Isotopes? A Straightforward Definition
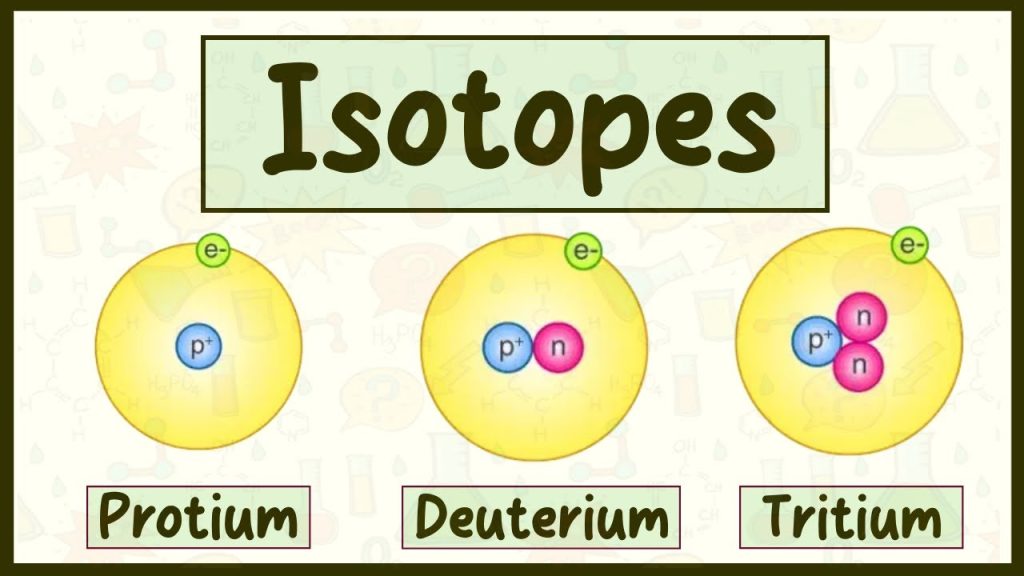
At its core, isotopes are variants of a particular chemical element that have the same number of protons but a different number of neutrons in their nuclei. This means that while isotopes of an element share many chemical properties, they differ in atomic mass and some physical properties.
Essential Characteristics of Isotopes
- Same Atomic Number: Isotopes of an element always have the same number of protons. For example, every carbon atom has 6 protons.
- Different Mass Numbers: Because isotopes differ in the number of neutrons, their mass numbers vary. Carbon-12 and Carbon-14 are both isotopes of carbon; however, Carbon-12 has 6 neutrons while Carbon-14 has 8.
- Chemical Similarity, Physical Differences: Despite having nearly identical chemical behavior, isotopes can exhibit differences in stability, radioactivity, and physical properties.
- Natural and Synthetic Occurrence: Some isotopes occur naturally (like Carbon-12), while others are artificially produced (such as Technetium-99m, widely used in medical imaging).
In summary, what is isotopes? They are different forms of an element’s atoms, distinguished primarily by variations in their neutron count, which can lead to remarkable differences in behavior and applications.
Historical and Contextual Background
The study of isotopes has a rich and fascinating history that has fundamentally changed our understanding of matter and the natural world. Let’s take a journey through time to see how the concept of isotopes evolved.
Early Discoveries and the Birth of Isotope Science
- Early Theories of Matter: Before the modern era, scientists believed that all atoms of a given element were identical. The idea that an element could exist in different forms was not yet conceived.
- Frederick Soddy and the Concept of Isotopes: In the early 20th century, British chemist Frederick Soddy observed that certain elements emitted different types of radiation despite having identical chemical properties. In 1913, he introduced the term “isotope” (from the Greek “isos,” meaning equal, and “topos,” meaning place) to describe these different forms that occupied the same position in the periodic table.
- The Role of Radiochemistry: The discovery of radioactivity and the subsequent research in radiochemistry played a key role in identifying and understanding isotopes. Scientists began to realize that the differences in atomic mass among isotopes could affect their stability and behavior.
Milestones in Isotope Research
- The Discovery of Carbon-14: In the 1940s, the development of radiocarbon dating by Willard Libby revolutionized archaeology and geology. Carbon-14, a radioactive isotope of carbon, allowed scientists to determine the age of ancient artifacts and geological formations.
- Nuclear Reactions and Artificial Isotopes: With the advent of nuclear reactors and particle accelerators, scientists began producing synthetic isotopes. These artificial isotopes have since become invaluable in various fields, including medicine, industry, and research.
- Modern Advances: Recent decades have seen the development of highly specialized isotope separation techniques and advanced mass spectrometry, further expanding our ability to study and utilize isotopes in diverse applications.
Notable Historical Anecdotes
- Frederick Soddy’s Breakthrough: Soddy’s work not only introduced the concept of isotopes but also reshaped our understanding of atomic structure, paving the way for modern nuclear science.
- Carbon Dating Revolution: The discovery and application of Carbon-14 dating transformed the field of archaeology, enabling researchers to accurately date ancient relics and revolutionize our understanding of human history.
In-Depth Exploration: Key Aspects and Types of Isotopes
To answer what is isotopes fully, we must delve into the different types, properties, and applications of isotopes. This section breaks down these concepts into manageable parts.
1. Types of Isotopes
Isotopes can generally be classified into two categories based on their stability:
a. Stable Isotopes
- Definition: Stable isotopes do not undergo radioactive decay over time. They remain unchanged and are found naturally in the environment.
- Examples:
- Carbon-12: The most common and stable isotope of carbon.
- Oxygen-16: The most abundant oxygen isotope.
- Applications: Stable isotopes are used in environmental studies, climate research, and metabolic studies. For example, the ratio of oxygen isotopes in ice cores can provide insights into historical climate conditions.
b. Radioactive (Unstable) Isotopes
- Definition: Radioactive isotopes are unstable and decay over time, emitting radiation in the process. Their decay rates are characterized by their half-lives.
- Examples:
- Carbon-14: Used in radiocarbon dating to determine the age of organic materials.
- Uranium-235: A key isotope used in nuclear reactors and weapons.
- Applications: Radioactive isotopes are widely used in medicine (for diagnostics and treatment), industry (for quality control and material analysis), and research (for tracing and dating).
2. Properties and Behavior of Isotopes
Understanding what is isotopes also requires knowing how their properties differ due to variations in neutron count:
a. Atomic Mass
- Explanation: The atomic mass of an isotope is the sum of its protons and neutrons. Different isotopes of the same element have different atomic masses.
- Impact: This difference in mass can affect the physical properties of the element, such as its density and behavior during chemical reactions.
b. Radioactivity
- Explanation: Radioactive isotopes decay at a predictable rate, emitting radiation. This property is quantified by the isotope’s half-life.
- Impact: Radioactivity can be harnessed for beneficial applications (e.g., medical imaging) or can pose hazards if not managed properly.
c. Chemical Behavior
- Explanation: Despite differences in mass and stability, isotopes of an element generally exhibit similar chemical behavior because they have the same number of electrons.
- Impact: This similarity is why isotopes can be used as tracers in chemical reactions or biological processes without significantly altering the reaction’s outcome.
3. The Role of Isotopes in Various Scientific Fields
Isotopes have become indispensable in multiple areas of research and industry:
a. Medicine
- Diagnostic Imaging: Radioactive isotopes like Technetium-99m are used in nuclear medicine to produce detailed images of the body’s internal structures.
- Cancer Treatment: Radioisotopes are used in radiotherapy to target and destroy cancerous cells.
- Tracer Studies: Stable isotopes help track metabolic processes and diagnose diseases.
b. Environmental Science
- Climate Research: The analysis of stable oxygen and hydrogen isotopes in ice cores and ocean sediments provides insights into past climate conditions.
- Pollution Tracking: Isotopic analysis helps identify sources of pollution and trace the movement of contaminants through ecosystems.
c. Archaeology and Geology
- Radiocarbon Dating: Carbon-14 dating has revolutionized archaeology by providing accurate age estimates for organic materials.
- Geochemical Tracing: Isotopic signatures help determine the origins of rocks, minerals, and even ancient human migrations.
d. Industry and Energy
- Nuclear Power: Isotopes like Uranium-235 and Plutonium-239 are critical for nuclear reactors, providing energy through controlled nuclear fission.
- Material Testing: Radioisotopes are used in industrial radiography to detect flaws in materials and structures.
4. Case Study: Carbon-14 Dating in Archaeology
Overview: Carbon-14 is a radioactive isotope of carbon that decays over time. Its predictable half-life allows scientists to determine the age of formerly living things.
- Process: Living organisms constantly exchange carbon with the environment. When they die, they stop absorbing carbon, and the Carbon-14 they contain begins to decay.
- Application: By measuring the remaining Carbon-14 in an archaeological sample, researchers can estimate when the organism died.
- Impact: This technique has been instrumental in dating ancient artifacts, understanding historical timelines, and reconstructing past environments.
5. The Isotope Effect in Chemistry
The differences between isotopes can also lead to subtle variations in chemical reactions, a phenomenon known as the isotope effect. This effect is particularly important in:
- Kinetic Studies: The rate at which a chemical reaction occurs can vary slightly depending on the isotopes involved.
- Mechanistic Insights: Studying these differences helps chemists understand reaction mechanisms and the behavior of molecules under different conditions.
Importance, Applications, and Benefits of Understanding Isotopes
Understanding what is isotopes is not just an academic exercise—it has practical implications that touch many aspects of modern life. Here are some of the key benefits and applications:
1. Advancing Scientific Research
- Improved Accuracy: Isotopes provide precise tools for dating, tracing, and analyzing natural processes.
- Enhanced Understanding: They allow researchers to unravel complex mechanisms in chemistry, biology, and geology, leading to breakthroughs in various scientific fields.
2. Medical Advancements
- Diagnostic Tools: Radioactive isotopes have revolutionized medical imaging and diagnostics, enabling early detection of diseases and improving treatment outcomes.
- Targeted Therapy: The ability to deliver radiation directly to diseased cells has made isotopes a cornerstone of modern cancer treatment.
3. Environmental and Climate Studies
- Historical Climate Reconstruction: Isotopic analysis of ice cores, sediments, and tree rings provides valuable data on historical climate patterns.
- Pollution Monitoring: Tracking isotopic signatures helps identify pollution sources and assess environmental impact, supporting efforts to mitigate ecological damage.
4. Industrial and Energy Applications
- Nuclear Energy: Isotopes are critical to the generation of nuclear power, which provides a significant portion of the world’s electricity.
- Quality Control: In manufacturing, isotopes are used for non-destructive testing and material analysis, ensuring product quality and safety.
5. Educational and Economic Impact
- STEM Education: Learning about isotopes fosters critical thinking and scientific literacy, essential for future innovations.
- Economic Benefits: The applications of isotopes in energy, medicine, and environmental management contribute significantly to economic growth and sustainability.
Addressing Common Misconceptions and FAQs
Even though isotopes play a pivotal role in science and technology, several misconceptions persist about what is isotopes. Let’s clear up some of these misunderstandings:
Misconception 1: All Isotopes Are Radioactive
- Clarification: Only a subset of isotopes is radioactive. Many isotopes are stable and do not undergo radioactive decay.
Misconception 2: Isotopes Change the Chemical Behavior of an Element
- Clarification: Isotopes of an element have nearly identical chemical properties because they have the same number of electrons. Their differences lie mainly in physical properties such as mass and stability.
Misconception 3: Isotopes Are Only Important in Nuclear Science
- Clarification: While isotopes are critical in nuclear science, they have diverse applications in medicine, environmental studies, archaeology, and industry.
Frequently Asked Questions (FAQs)
Q1: What is isotopes in simple terms?
A1: Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, leading to differences in atomic mass and stability.Q2: Why are isotopes important in dating ancient artifacts?
A2: Radioactive isotopes like Carbon-14 decay at a known rate, allowing scientists to estimate the age of organic materials based on the remaining amount of the isotope.Q3: Can isotopes affect the outcome of chemical reactions?
A3: While isotopes do not significantly change the chemical properties of an element, subtle differences in mass can influence reaction rates—a phenomenon known as the isotope effect.Q4: Are synthetic isotopes harmful?
A4: Some synthetic isotopes are radioactive and must be handled with care; however, many have beneficial applications in medicine and industry when used safely under controlled conditions.Q5: How do scientists detect and measure isotopes?
A5: Techniques such as mass spectrometry, nuclear magnetic resonance (NMR), and radiometric dating are used to detect and measure isotopes with high precision.
Modern Relevance and Current Trends
Isotope research and application continue to evolve, reflecting ongoing advances in technology and expanding our understanding of natural processes. Here are some of the current trends and future directions related to what is isotopes:
1. Advances in Analytical Techniques
- Mass Spectrometry Improvements: Cutting-edge mass spectrometers are providing unprecedented precision in isotope measurement, enabling breakthroughs in fields from geochemistry to forensic science.
- Isotope Ratio Analysis: Enhanced analytical methods allow for detailed studies of isotope ratios, crucial for climate research, food authenticity verification, and tracking pollution sources.
2. Medical and Biomedical Innovations
- Targeted Radiotherapy: New radioactive isotopes are being developed for more precise cancer treatments that minimize damage to healthy tissue.
- Diagnostic Imaging: Advances in nuclear imaging techniques, powered by isotopes such as Technetium-99m, continue to improve the accuracy and safety of diagnostic procedures.
3. Environmental Monitoring and Climate Change
- Climate Reconstruction: Improved isotopic analysis of ice cores, sediments, and atmospheric samples is providing deeper insights into past climate fluctuations, helping predict future trends.
- Ecosystem Studies: Stable isotopes are increasingly used to track nutrient cycles and assess the health of ecosystems, informing conservation strategies and environmental policies.
4. Nuclear Energy and Waste Management
- Safer Reactors: Research into the behavior of isotopes is essential for designing safer nuclear reactors and more effective waste management systems.
- Isotope Production: Innovations in isotope production are making it easier to obtain rare isotopes needed for energy, medicine, and scientific research.
5. Cross-Disciplinary Applications
- Archaeological Forensics: Isotope analysis is being used to uncover migration patterns and dietary habits in ancient populations.
- Material Science: In industries ranging from aerospace to consumer electronics, isotopic labeling and tracing help optimize manufacturing processes and improve product quality.
The Practical Benefits of Understanding Isotopes
A thorough grasp of what is isotopes offers many practical benefits:
1. Enhanced Scientific Research and Innovation
- Precision Measurements: Accurate isotope analysis underpins research in diverse scientific fields, leading to discoveries that can improve our understanding of everything from climate change to human biology.
- Innovative Applications: New uses for isotopes—such as in targeted cancer therapies or advanced materials testing—drive innovation and technological progress.
2. Improved Healthcare and Medical Treatments
- Early Diagnosis and Treatment: Radiological techniques that use isotopes enable early detection of diseases and more effective treatments.
- Personalized Medicine: Isotopic analysis can help tailor treatments to individual patients, improving outcomes and reducing side effects.
3. Better Environmental and Resource Management
- Tracking Environmental Changes: Isotopic data help scientists monitor pollution, understand nutrient cycles, and manage natural resources more effectively.
- Sustainable Practices: Improved understanding of isotope behavior supports more sustainable practices in agriculture, water management, and energy production.
4. Economic and Industrial Advantages
- Efficiency in Production: In manufacturing and energy sectors, isotopes are used for quality control and process optimization, resulting in cost savings and improved efficiency.
- Market Competitiveness: Industries that leverage isotope technology gain a competitive edge through enhanced product reliability and innovative services.
Conclusion: Embracing the World of Isotopes
Throughout this comprehensive exploration, we have unraveled the intriguing question: what is isotopes? We’ve defined isotopes as variants of elements that differ in neutron number, explored their rich historical background, and delved into their diverse types and applications. From revolutionizing archaeology through radiocarbon dating to powering modern nuclear medicine and energy production, isotopes are a cornerstone of scientific progress and practical innovation.
Key Takeaways:
- Definition: Isotopes are forms of a chemical element that have the same number of protons but different numbers of neutrons, leading to differences in atomic mass and physical properties.
- Historical Evolution: The concept of isotopes emerged from early 20th-century discoveries in radiochemistry, reshaping our understanding of atomic structure and laying the foundation for modern nuclear science.
- Types and Properties: With stable and radioactive variants, isotopes have unique characteristics that influence their behavior and applications.
- Real-World Applications: Isotopes play a vital role in medicine, environmental science, archaeology, industry, and more.
- Modern Relevance: Ongoing research and technological advances continue to expand our ability to measure, utilize, and manage isotopes, driving progress across many fields.
- Practical Benefits: A deep understanding of isotopes enhances scientific research, healthcare, environmental management, and industrial efficiency.
As you reflect on this guide, consider the myriad ways isotopes influence our world—from the age of ancient artifacts to cutting-edge medical treatments. Embracing the science of isotopes not only deepens your understanding of matter itself but also opens the door to innovative applications that can transform lives and industries.
Call to Action:
- Join the Conversation: Share your thoughts, questions, or experiences with isotopes in the comments below. How have isotopes impacted your field of interest or work?
- Share This Post: If you found this guide insightful, please share it on social media or with colleagues, friends, and anyone interested in the wonders of science.
- Keep Exploring: Continue your journey into the fascinating realm of chemistry and nuclear science by exploring additional resources, online courses, and related articles on isotopic applications and innovations.
Additional Resources
For further reading and exploration of what is isotopes, consider these reputable sources:
- Encyclopedia Britannica – Isotope
- U.S. Nuclear Regulatory Commission – Isotopes in Medicine
- MIT OpenCourseWare – Nuclear Chemistry
- American Chemical Society – Isotope Research
- Radiocarbon Dating – The Basics by the National Oceanic and Atmospheric Administration (NOAA)
Final Thoughts
Isotopes are much more than just a scientific curiosity—they are the subtle variations in nature that allow us to unlock the mysteries of our past, diagnose and treat diseases, and drive technological innovation. By understanding what is isotopes, we gain valuable insights into the very fabric of matter and the processes that govern our world. Whether you are a seasoned scientist, a student, or someone with a budding interest in the sciences, embracing the concept of isotopes enriches your knowledge and empowers you to see the world in a new light.
Thank you for joining us on this in-depth exploration of isotopes. Stay curious, keep learning, and let the fascinating world of isotopes inspire you to explore the unseen wonders of our universe.