“What Is a Solvent? Everything You Need to Know”
Have you ever wondered how your morning coffee stays perfectly mixed, or why the salt you add to a recipe seems to vanish into your water? These everyday phenomena are made possible by the fascinating world of solutions—and at the heart of every solution is a key player known as the solvent. But what is a solvent? In this comprehensive guide, we’ll explore the concept of a solvent from multiple angles: its definition, its essential characteristics, its historical evolution, and its myriad applications in science, industry, and everyday life. Whether you’re a student, a professional, or simply a curious mind, this article will equip you with everything you need to know about what is a solvent and why it is so important.
In this post, we will cover:
- A Clear Definition: A concise yet thorough explanation of what a solvent is, along with its essential properties.
- Historical and Contextual Background: The origins and evolution of the concept of a solvent, with notable milestones and historical anecdotes.
- In-Depth Exploration: A breakdown of key points, attributes, and categories of solvents. We’ll examine examples from chemistry, real-world applications, and case studies to illustrate how solvents are used.
- Importance, Applications, and Benefits: Why understanding what is a solvent matters in everyday life, society, science, business, and beyond.
- Common Misconceptions and FAQs: Clarification of common myths and misunderstandings surrounding solvents, with a helpful Q&A section.
- Modern Relevance and Current Trends: An exploration of recent developments and debates regarding solvents in today’s world.
- Conclusion and Call-to-Action: A succinct summary of key insights and an invitation to further explore, discuss, and apply this knowledge.
By the end of this guide, you will have a thorough understanding of what is a solvent—its scientific definition, practical applications, and critical role in shaping our material world. Let’s dive in and explore the dynamic and indispensable role that solvents play!
Introduction: The Unsung Hero of Mixtures
Imagine a world without solvents. Without them, your favorite beverage would be a jumbled mess of undissolved ingredients, medicines would be less effective, and industrial processes might come to a grinding halt. Solvents are the “invisible” heroes that enable substances to mix, react, and function in countless ways.
An Intriguing Hook
Did you know that water, the most common solvent on Earth, is often called the “universal solvent”? This is because water can dissolve more substances than any other liquid, making it crucial not only for life but also for industrial processes, environmental systems, and everyday household tasks. From the chemistry lab to your kitchen, solvents are everywhere—and understanding what is a solvent is key to unlocking the mysteries behind how mixtures work.
What This Post Will Cover and Why It Matters
In today’s post, we will explore:
- Definition and Characteristics: What exactly is a solvent? We’ll break down its essential traits in simple, clear language.
- Historical Background: Discover the origins of the concept, tracing its evolution from early alchemy and scientific inquiry to modern chemistry.
- Types of Solvents: Learn about the different kinds of solvents—polar, non-polar, organic, inorganic—and see real-world examples and case studies.
- Applications and Benefits: Understand the role of solvents in everyday life, from cooking and cleaning to advanced industrial and medical applications.
- Common Misconceptions and FAQs: Get answers to frequently asked questions and clear up any myths about solvents.
- Modern Trends and Developments: Explore how new research and technology are reshaping our understanding of solvents and their applications in today’s digital, globalized world.
Understanding what is a solvent not only deepens our appreciation for the science behind everyday phenomena but also equips us with the knowledge to make informed decisions in areas ranging from health and safety to innovation and sustainability.
What Is a Solvent? A Straightforward Definition
Defining a Solvent
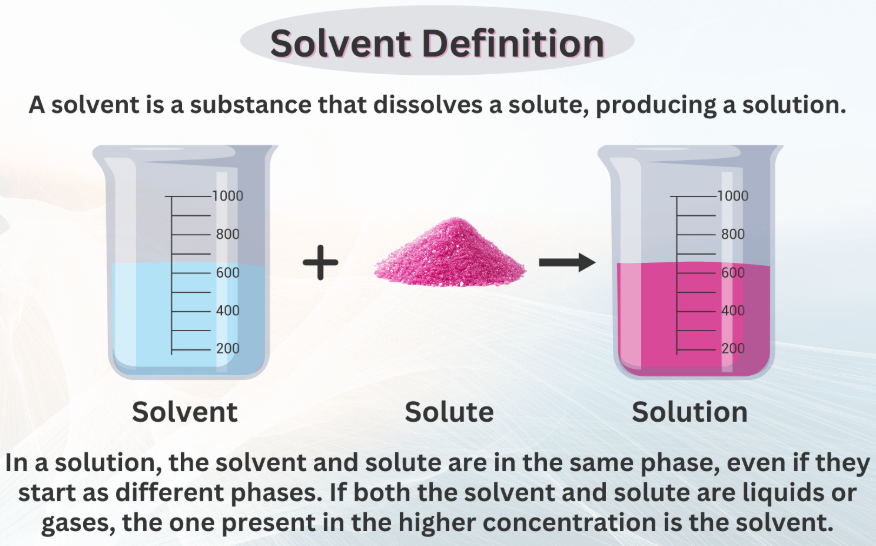
At its most fundamental level, what is a solvent? In the field of chemistry, a solvent is a substance—typically a liquid—that has the ability to dissolve a solute (another substance) to form a homogeneous mixture known as a solution. The solvent is usually present in a greater amount than the solute, and its properties largely determine the behavior and characteristics of the resulting solution.
Essential Characteristics of a Solvent
To fully understand what is a solvent, consider the following defining characteristics:
Dissolving Power:
The primary function of a solvent is to dissolve other substances (solutes) to form a solution. For instance, water dissolves salt to create saline.Homogeneity:
In a solution, the solvent ensures that the solute is uniformly distributed at a molecular level. This homogeneity is critical for consistent chemical reactions and product quality.Physical Properties:
Solvents are characterized by properties such as boiling point, melting point, polarity, viscosity, and density. These properties affect how well a solvent can dissolve a given solute. For example, polar solvents like water are effective at dissolving other polar substances.Role as a Medium:
In many chemical reactions, the solvent serves as a medium in which reactants interact. The choice of solvent can significantly influence reaction rates, yields, and even the mechanism of the reaction.Versatility:
Solvents are not limited to a single type of substance. They can be organic (e.g., ethanol, acetone) or inorganic (e.g., water) and are used in a wide variety of applications from cleaning and extraction to manufacturing and pharmaceuticals.
By grasping these core characteristics, you now have a clear answer to the question: what is a solvent? It is the crucial component in mixtures that enables solutes to be evenly distributed and facilitates a host of chemical processes.
Historical and Contextual Background
The Evolution of the Concept of a Solvent
The concept of a solvent has evolved significantly over the centuries, influencing and being influenced by the development of modern science.
Early Beginnings and Alchemical Roots
Ancient and Medieval Practices:
The idea of dissolving substances dates back to ancient civilizations. Early alchemists experimented with various liquids to transform or purify substances, laying the groundwork for what we now understand as solvents. Although alchemy was shrouded in mysticism, it contributed to the gradual shift toward a scientific understanding of matter.Transition from Alchemy to Chemistry:
During the 17th and 18th centuries, the transition from alchemy to modern chemistry brought about a more systematic study of substances and their interactions. Pioneers such as Robert Boyle and Antoine Lavoisier began to analyze how substances dissolved in liquids, paving the way for the concept of a solvent in scientific terms.
Scientific Milestones in Understanding Solvents
Development of the Concept of Solutions:
In the 19th century, as chemists began to study solutions more rigorously, they developed the idea that a solvent is the major component in a solution. John Dalton’s atomic theory and subsequent advances in molecular theory further refined our understanding of how solvents work at a molecular level.Advancements in Analytical Chemistry:
The advent of techniques such as spectroscopy, chromatography, and later, nuclear magnetic resonance (NMR), allowed scientists to study solutions in detail. These technological advancements led to a deeper understanding of solvent properties, interactions, and applications in various industries.
Notable Historical Anecdotes
Water as the Universal Solvent:
Water’s reputation as the “universal solvent” has deep historical roots. Ancient civilizations recognized water’s unique ability to dissolve a vast array of substances, which made it indispensable for both daily life and early scientific inquiry.Industrial Revolution:
The Industrial Revolution saw a surge in the use of solvents in manufacturing and chemical production. Solvents became critical for processes such as painting, cleaning, and the production of polymers—laying the foundation for modern industrial chemistry.
This rich historical context illustrates how our understanding of what is a solvent has evolved from early mystical practices to a cornerstone of modern science and industry.
In-Depth Exploration: Types, Attributes, and Categories of Solvents
To fully appreciate what is a solvent, we must delve into its various forms and applications. Solvents play diverse roles across many fields, and here we explore their key types and attributes.
1. Polar and Non-Polar Solvents
Polar Solvents
- Definition:
Polar solvents have molecules with a significant dipole moment due to uneven distribution of electrons, making them effective at dissolving ionic and other polar substances. - Examples:
- Water: The quintessential polar solvent, known for its ability to dissolve salts, sugars, and many organic compounds.
- Ethanol: A polar solvent used widely in laboratories, pharmaceuticals, and as a disinfectant.
- Characteristics:
- High Dielectric Constant: This allows polar solvents to stabilize ions in solution.
- Hydrogen Bonding: Many polar solvents, particularly water, can form hydrogen bonds, enhancing their solvency.
Non-Polar Solvents
- Definition:
Non-polar solvents have molecules with a more symmetrical distribution of electrons, making them ideal for dissolving non-polar substances. - Examples:
- Hexane: Commonly used in the extraction of oils and fats.
- Benzene: Historically used in industrial applications, though its use has diminished due to health concerns.
- Characteristics:
- Low Dielectric Constant: Non-polar solvents do not stabilize ions, which makes them poor at dissolving polar substances.
- Van der Waals Forces: The interactions between non-polar molecules are mainly due to dispersion forces.
2. Organic and Inorganic Solvents
Organic Solvents
- Definition:
Organic solvents are typically carbon-based compounds that can dissolve a wide range of substances. They are often volatile and flammable. - Examples:
- Acetone: Used in nail polish remover and as a cleaning agent.
- Toluene: Commonly used in paints, adhesives, and chemical synthesis.
- Attributes:
- Volatility: Many organic solvents evaporate quickly, making them useful for applications requiring rapid drying.
- Chemical Reactivity: Their molecular structure can be tailored to dissolve specific substances, enhancing their utility in chemical processes.
Inorganic Solvents
- Definition:
Inorganic solvents, most notably water, are not based on carbon compounds and are essential for many biological and chemical processes. - Examples:
- Water: The most widely used inorganic solvent, essential for life and numerous industrial processes.
- Liquid Ammonia: Used as a solvent in certain chemical reactions, particularly in the production of synthetic materials.
- Attributes:
- Environmental Compatibility: Inorganic solvents like water are often more environmentally friendly compared to many organic solvents.
- Versatility: Their properties, such as high heat capacity and surface tension, make them valuable in various applications.
3. Special-Use Solvents
Ionic Liquids
- Definition:
Ionic liquids are salts in the liquid state, typically composed of organic cations and inorganic or organic anions. They have unique properties such as low volatility and high thermal stability. - Applications:
- Green Chemistry: Used as environmentally friendly alternatives to traditional solvents in chemical synthesis.
- Energy Storage: Applied in the development of batteries and fuel cells due to their stability and conductivity.
Supercritical Fluids
- Definition:
Supercritical fluids are substances that are above their critical temperature and pressure, exhibiting properties of both liquids and gases. Carbon dioxide is a common example. - Applications:
- Extraction Processes: Widely used in decaffeination of coffee and extraction of essential oils.
- Advanced Material Processing: Employed in processes such as polymer formation and nanoparticle synthesis.
4. Real-World Examples and Case Studies
Case Study 1: Water as the Universal Solvent
- Overview:
Water’s ability to dissolve a vast array of substances is fundamental to life on Earth. From its role in bodily functions to its use in industrial processes, water is indispensable. - Key Points:
- Biological Importance: Facilitates chemical reactions in living organisms.
- Industrial Applications: Used in cooling systems, chemical reactions, and as a cleaning agent.
- Impact:
Water’s properties as a solvent have made it a subject of extensive study and a critical resource in every aspect of life.
Case Study 2: Organic Solvents in Industrial Processes
- Overview:
Organic solvents like acetone and toluene play pivotal roles in manufacturing, cleaning, and chemical synthesis. - Key Points:
- Application in Manufacturing: Used in the production of plastics, paints, and adhesives.
- Environmental Concerns: Their volatility and toxicity have led to strict regulations and a push toward greener alternatives.
- Impact:
The development of safer organic solvents and alternatives like ionic liquids is reshaping industrial processes and reducing environmental impact.
Case Study 3: Technological Innovations with Special-Use Solvents
- Overview:
The rise of ionic liquids and supercritical fluids represents the cutting edge of solvent technology. - Key Points:
- Advances in Green Chemistry: Ionic liquids offer a sustainable alternative to traditional volatile organic compounds.
- Applications in Energy: Supercritical carbon dioxide is used in energy storage and extraction processes.
- Impact:
These innovations are not only advancing scientific research but also paving the way for environmentally friendly industrial practices.
Importance, Applications, and Benefits of Understanding What Is a Solvent
Understanding what is a solvent is crucial across many domains. Here’s why:
1. Enhancing Scientific and Industrial Processes
- Chemical Reactions:
Solvents are essential in carrying out chemical reactions efficiently. They provide a medium in which reactants can mix, interact, and transform. - Product Consistency:
In manufacturing, the use of a consistent solvent ensures that products—from pharmaceuticals to paints—meet quality standards. - Innovative Research:
Understanding solvent properties drives innovations in fields like materials science, nanotechnology, and biotechnology.
2. Everyday Life and Practical Applications
- Household Uses:
Many household products, such as cleaning agents, cosmetics, and even beverages, rely on solvents to achieve their desired properties. - Health and Safety:
Knowing about solvents can help individuals make informed choices about the products they use. For example, understanding the differences between organic and inorganic solvents can guide you toward safer, more environmentally friendly options. - Cooking and Food Preparation:
In the culinary world, solvents play a role in processes such as extraction of flavors and emulsification. Recognizing the principles behind these processes can enhance your cooking skills.
3. Environmental and Sustainability Impacts
- Green Chemistry:
The development of eco-friendly solvents is critical for reducing industrial pollution and conserving natural resources. - Waste Reduction:
Efficient solvent use in processes like recycling and waste treatment can minimize environmental impact. - Renewable Energy:
Solvents are used in renewable energy technologies, such as solar cells and batteries, making them key components in the transition to a sustainable future.
4. Educational and Cognitive Benefits
- STEM Education:
The concept of a solvent is fundamental in chemistry and physics curricula. Learning about solvents improves your understanding of chemical properties and processes. - Critical Thinking:
Analyzing the role and behavior of solvents in different contexts can enhance your problem-solving skills and scientific literacy. - Interdisciplinary Learning:
The study of solvents bridges disciplines—from chemistry and biology to environmental science and engineering—fostering a holistic approach to learning.
5. Business and Technological Advancements
- Innovation in Product Development:
Companies rely on solvents to develop new products and improve existing ones. Whether in the production of high-performance materials or in drug formulation, solvents are key to innovation. - Operational Efficiency:
Understanding and optimizing solvent use can lead to cost savings and improved efficiency in industrial processes. - Competitive Advantage:
Businesses that invest in environmentally friendly solvent technologies often gain a competitive edge in markets that increasingly value sustainability and social responsibility.
Addressing Common Misconceptions and FAQs
Despite the widespread importance of solvents, there are several misconceptions about what is a solvent. Let’s address these common myths and answer frequently asked questions.
Misconception 1: A Solvent Is Just Any Liquid
Myth:
Many people assume that any liquid can be considered a solvent.
Reality:
- Specific Properties Matter:
Not all liquids have the ability to dissolve substances effectively. A solvent must have specific properties, such as appropriate polarity, boiling point, and chemical stability, to function as intended. - Context-Specific Use:
For instance, while water is an excellent solvent for many substances due to its polarity, it is not effective for dissolving non-polar compounds like oils. Understanding these distinctions is key.
Misconception 2: The Only Solvent Worth Knowing Is Water
Myth:
Water is often dubbed the “universal solvent,” leading some to believe it is the only solvent of practical importance.
Reality:
- Diverse Applications:
Although water is crucial, there are many other solvents—both organic and inorganic—that play critical roles in various industries. Organic solvents like ethanol, acetone, and benzene each have unique properties that make them indispensable in specific contexts. - Tailored Solutions:
Different chemical processes require different solvents. The choice of solvent can affect reaction rates, product purity, and environmental impact.
Misconception 3: Solvent Use Is Static and Unchanging
Myth:
Some believe that the methods and technologies for using solvents have remained the same over time.
Reality:
- Technological Evolution:
Advances in science and technology continuously lead to new solvent formulations and innovative applications. From ionic liquids and supercritical fluids to greener, more sustainable options, the field is dynamic and ever-evolving. - Interdisciplinary Research:
Ongoing research in chemistry, environmental science, and engineering is constantly expanding our understanding of solvents and their potential.
Frequently Asked Questions (FAQs)
Q: What is a solvent?
A: A solvent is a substance, typically a liquid, that dissolves a solute to form a homogeneous solution. It plays a key role in chemical reactions, product formulation, and many industrial processes.Q: Why is water often called the “universal solvent”?
A: Water is referred to as the “universal solvent” because of its exceptional ability to dissolve a wide range of substances, owing to its polar nature and hydrogen-bonding capabilities.Q: Can solvents be used for more than just dissolving substances?
A: Yes. In addition to forming solutions, solvents are integral to many processes, including chemical reactions, extraction, cleaning, and even as a medium for energy storage and delivery in various technologies.Q: How do I choose the right solvent for a particular application?
A: The choice of solvent depends on the chemical properties of the solute, the desired concentration, the reaction conditions, and environmental or safety considerations. Consulting material safety data sheets (MSDS) and scientific literature can provide guidance.Q: Are there environmentally friendly solvents available?
A: Absolutely. The field of green chemistry is dedicated to developing eco-friendly solvents, such as water-based solutions, ionic liquids, and biodegradable organic solvents, to reduce environmental impact.
Modern Relevance and Current Trends
Solvents in Today’s Digital, Industrial, and Environmental Landscape
The concept of a solvent is as relevant today as it was centuries ago, playing a critical role in innovation, sustainability, and everyday life. Here are some modern trends and developments that illustrate what is a solvent in our current world.
Technological Innovations and Advanced Solvents
- Digital Manufacturing:
Advances in digital manufacturing and 3D printing often rely on specialized solvents to prepare materials, clean equipment, and facilitate the printing process. The development of precision solvents has enabled higher quality production and more innovative product designs. - Nanotechnology and Materials Science:
In the realm of nanotechnology, solvents are used to disperse nanoparticles and create novel materials with unique properties. Research into nanofluidics and solvent-based self-assembly is pushing the boundaries of what’s possible in materials science. - Energy Storage and Renewable Technologies:
Innovative solvent systems are critical in the development of next-generation batteries, fuel cells, and solar energy devices. For instance, specialized electrolytes—often formulated as solvents—are central to improving the performance and safety of lithium-ion batteries.
Environmental and Sustainability Trends
- Green Chemistry:
There is a growing emphasis on developing sustainable and environmentally benign solvents. Researchers are working on solvent alternatives that reduce toxicity and environmental harm, which is essential for industries such as pharmaceuticals, coatings, and manufacturing. - Recycling and Waste Reduction:
Solvents play a crucial role in recycling processes and in the treatment of industrial waste. Techniques that optimize solvent recovery and reuse are critical for reducing waste and lowering production costs. - Sustainable Agriculture:
In agriculture, environmentally friendly solvents are used in the production of pesticides, fertilizers, and herbicides, ensuring that these products are effective while minimizing ecological impact.
Business, Education, and Cultural Impact
- Innovation in Product Development:
In business, effective solvent use can be a competitive advantage. Companies that invest in advanced solvent technologies often see improvements in product quality, operational efficiency, and sustainability. - Educational Advances:
Modern chemistry and engineering curricula now emphasize green and sustainable chemistry, with solvents as a core topic. Students learn about solvent properties, selection criteria, and safe handling practices, equipping them with essential skills for future innovation. - Cultural Perception of Science:
Understanding solvents—and the science behind them—can also influence public perceptions of chemistry and technology. By demystifying complex concepts, educators and communicators help foster a more scientifically literate society.
Conclusion: The Power of Solutions in Our Material World
In exploring what is a solvent, we have journeyed through the science, history, and applications of these indispensable substances. A solvent is much more than just a liquid that dissolves another substance—it is the medium that enables reactions, drives industrial processes, and even supports life itself. Whether in a chemistry lab, a manufacturing plant, or your own kitchen, solvents are at the core of countless processes that shape our world.
Key Takeaways
- Definition and Core Characteristics:
A solvent is a substance that dissolves a solute to form a homogeneous solution. Essential traits include its dissolving power, homogeneity, and its role as a reaction medium. Whether in the context of chemistry, mathematics, or technology, understanding what is a solvent is fundamental. - Historical Evolution:
The concept of a solvent has evolved from early alchemical practices to modern scientific methods, reflecting centuries of human inquiry and innovation. - Diverse Manifestations:
Solvents come in various forms—polar, non-polar, organic, inorganic, and specialized types like ionic liquids and supercritical fluids—each suited to different applications. - Practical Applications and Benefits:
From ensuring the quality of consumer products and pharmaceuticals to driving advances in energy storage and green technology, solvents play a critical role in our daily lives and global industries. - Modern Trends:
The development of eco-friendly and high-performance solvents is a vibrant area of research and industry, with implications for sustainability, technological innovation, and economic growth.
Call-to-Action
Now that you have a comprehensive understanding of what is a solvent, it’s time to apply this knowledge:
- Reflect on Everyday Applications:
Next time you use a household cleaner or enjoy a glass of water, think about the role solvents play in making these everyday items possible. - Engage with Further Learning:
Dive deeper into the world of solvents by exploring additional resources. Visit reputable sites such as the American Chemical Society or Royal Society of Chemistry for more detailed information. - Share Your Thoughts:
We’d love to hear from you! Share your experiences with solvents—whether in the lab, industry, or everyday life—in the comments below. - Spread the Knowledge:
If you found this post informative, please share it with colleagues, students, or anyone interested in the fascinating science behind solutions and solvents. - Apply in Your Work:
For professionals and educators, consider how a deeper understanding of solvents can enhance your projects, research, or teaching methods. Use this knowledge to innovate and solve problems more effectively.
Final Thoughts
Understanding what is a solvent is more than an academic exercise—it is a gateway to appreciating the intricate processes that govern our material world. From the clear, life-sustaining role of water as a universal solvent to the cutting-edge applications of specialized solvents in technology and green chemistry, solvents are at the heart of countless scientific and industrial advancements. They remind us that even the simplest substances can have profound and far-reaching impacts.
As you go about your daily life, take a moment to appreciate the solvents that silently work to make everything function—from the water you drink and the medicines you rely on, to the innovative technologies that shape our future. Embrace the power of solutions, and let your understanding of what is a solvent inspire you to think critically, act sustainably, and continuously seek innovative answers to the challenges around you.
Thank you for joining us on this in-depth exploration of what is a solvent. We hope this guide has enriched your understanding, sparked your curiosity, and empowered you with practical insights. Happy exploring, and may your journey into the world of solvents lead you to new discoveries and endless possibilities!