Systems and Fundamental Forces
Introduction
Atoms are the smallest and most basic units of matter, serving as the defining structures of elements. Understanding the composition and behavior of atoms forms the foundation for quantum, atomic, and nuclear physics. In this lesson, we will explore the structure of atoms, atomic theory, electron energy levels, and the forces at play within atoms. By the end of this unit, you will be able to describe the internal structure of atoms and their nuclei.
Fundamental Particles
Atoms consist of three main particles:
| Particle | Symbol | Mass (kg) | Charge (C) |
|---|---|---|---|
| Electron | e⁻ | 9.11 × 10⁻³¹ | -1.6 × 10⁻¹⁹ |
| Proton | p | 1.673 × 10⁻²⁷ | 1.6 × 10⁻¹⁹ |
| Neutron | n | 1.673 × 10⁻²⁷ | No charge |
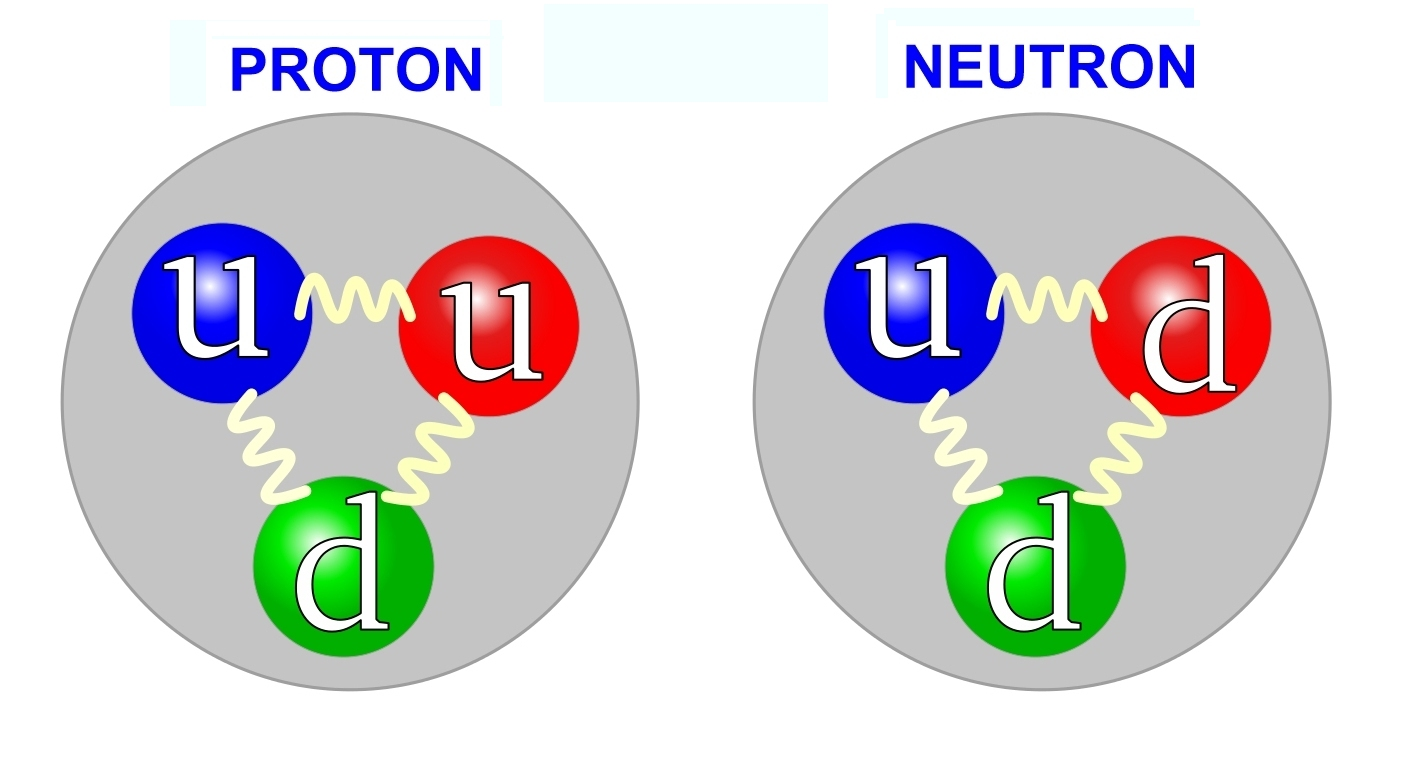
While electrons, neutrinos, photons, and quarks are considered fundamental particles (particles that cannot be broken down further), protons and neutrons are composed of quarks. These quarks carry fractional charges of the elementary charge of the electron:
Up, Charm, and Top Quarks: Fractional charge of +⅔.
Down, Strange, and Bottom Quarks: Fractional charge of −⅓.
Protons are made up of two up quarks and one down quark, while neutrons consist of two down quarks and one up quark.
Atomic Structure
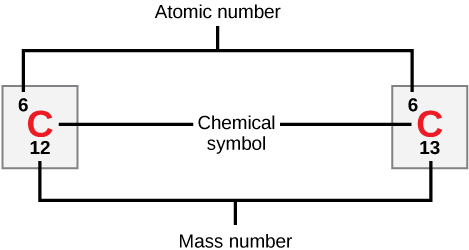
The nucleus of an atom, composed of protons and neutrons (collectively called nucleons), defines an element’s atomic number (Z). Adding the number of neutrons (N) and protons gives the element’s mass number (A):
A = Z + N
Atoms with the same number of protons but different numbers of neutrons are called isotopes. For example, carbon-12 and carbon-14 are isotopes of carbon, with six protons but differing numbers of neutrons.
Rutherford’s Model
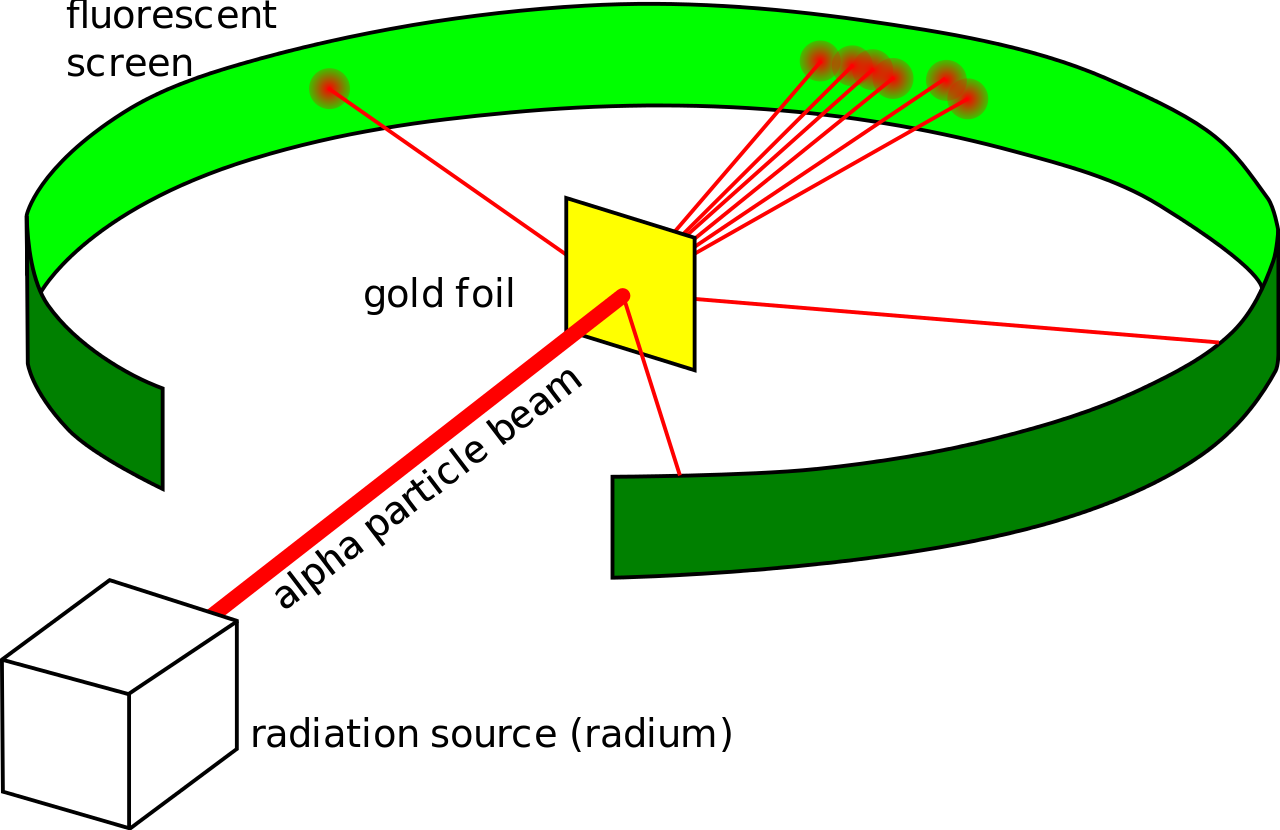
Ernest Rutherford’s famous gold foil experiment revolutionized our understanding of atomic structure. By directing alpha particles at a thin gold foil and observing their scattering patterns, Rutherford concluded:
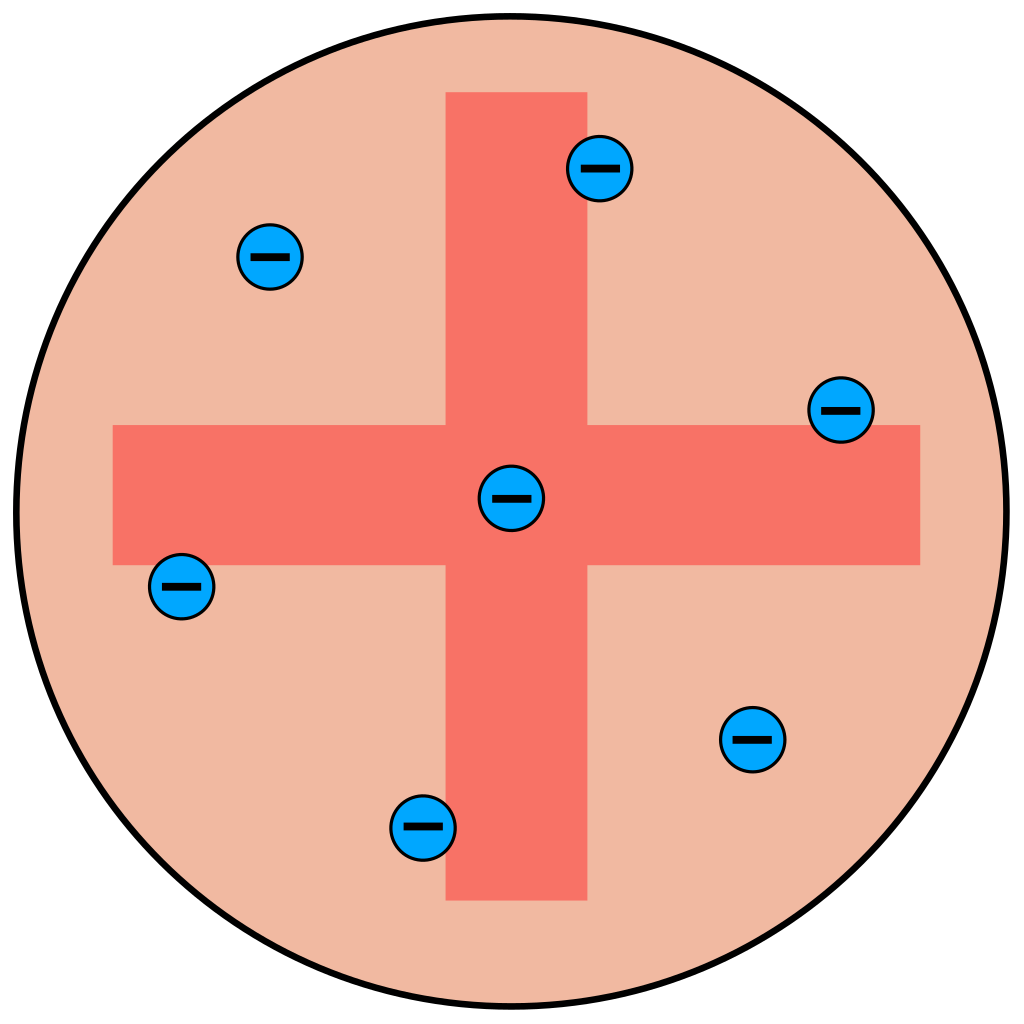
Atoms are mostly empty space.
The nucleus is a small, dense, positively charged region.
Electrons orbit the nucleus, much like planets orbit the Sun.
While groundbreaking, Rutherford’s model had its limitations:
It did not explain the discrete emission spectra observed in experiments.
It failed to account for why orbiting electrons do not radiate energy and spiral into the nucleus.
The Bohr Model
Niels Bohr refined Rutherford’s model by incorporating quantum theory. Key features of the Bohr Model include:
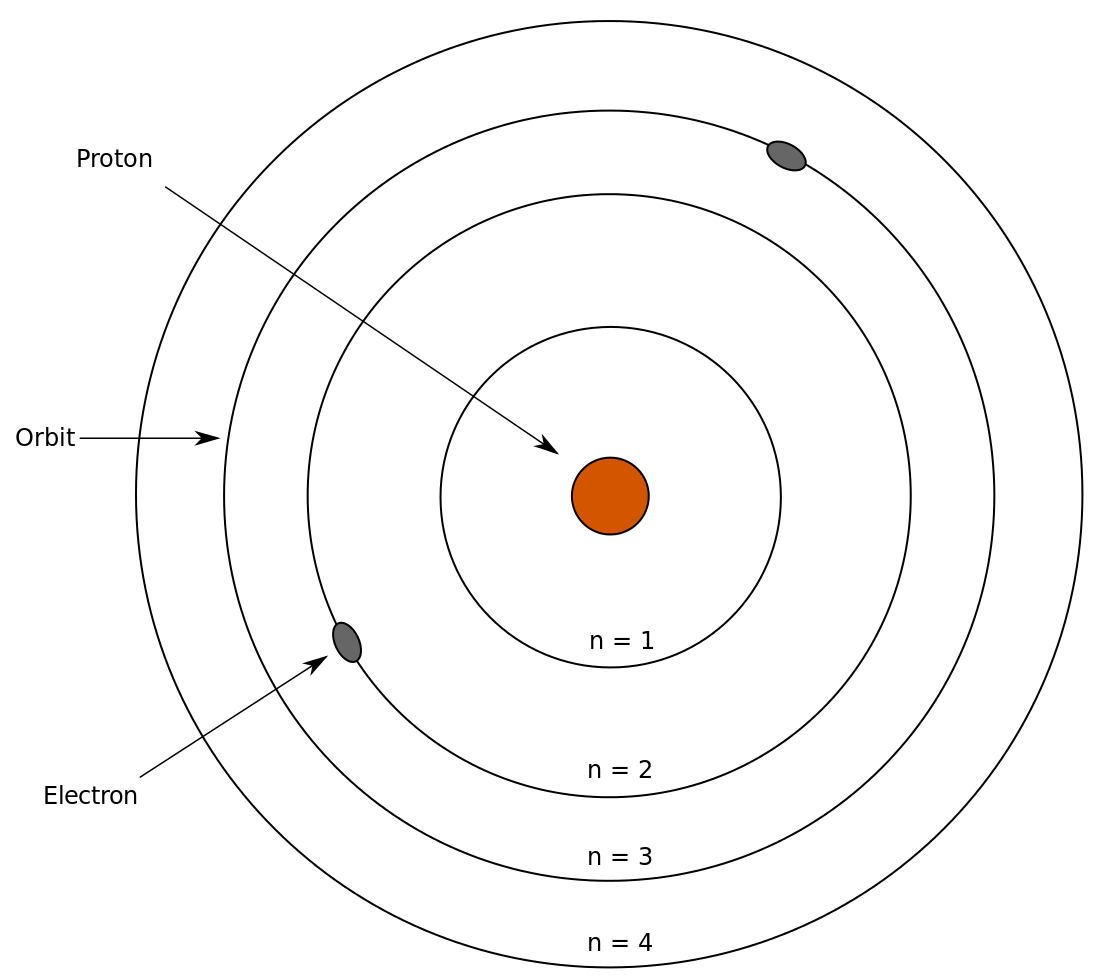
Electrons orbit the nucleus in fixed energy levels.
Electrons emit or absorb energy only when transitioning between these levels.
These transitions produce photons of specific wavelengths, explaining the discrete emission spectra.
For example, in the hydrogen atom, the energy of a photon emitted when an electron transitions from a higher energy level (n=j) to a lower one (n=i) is given by:
E = |E⁺ − E⁻|
The photon’s wavelength (λ) is:
λ = hc/E
where:
c = speed of light (3.00 × 10⁸ m/s),
h = Planck’s constant (6.63 × 10⁻³⁴ Js).
The Strong Nuclear Force
Despite the repulsive Coulomb forces between positively charged protons in the nucleus, atoms remain stable due to the strong nuclear force, the most powerful force in nature. This force binds protons and neutrons together, ensuring nuclear stability.
Practice Problem
Energy Levels in Hydrogen
Using the energy level diagram for hydrogen:
How much energy must a ground-state electron (n=1, E=-13.6 eV) absorb to reach the n=3 energy level (E=-1.51 eV)?
Solution: E⁻ − E⁺ = (-1.51 eV) − (-13.6 eV) = 12.09 eV.
An electron in the n=4 energy level can drop to n=3, n=2, or n=1. What are the wavelengths of the emitted photons for these transitions?
Solution:
E⁴ → E = 0.65 eV → λ = 1,910 nm (infrared).
E⁴ → E² = 2.55 eV → λ = 487 nm (visible light).
E⁴ → E¹ = 12.8 eV → λ = 97 nm (ultraviolet).
Additional Practice Questions
Hydrogen Spectra: Electrons starting in the 4th energy level and ending in the ground state can produce how many lines in the hydrogen spectra?
A) 7
B) 6
C) 5
D) 4
E) 3
Answer: B. Six transitions are possible.
Gold Foil Experiment: Rutherford concluded that most alpha particles pass through gold foil undeflected because:
A) The nucleus is positively charged.
B) Most atomic mass resides in the nucleus.
C) The nucleus contains protons and neutrons.
D) The nucleus is small compared to the atom.
Answer: D. Most of the atom is empty space.
Bohr Model: Classical physics predicts that electrons radiate energy while orbiting the nucleus. Why doesn’t this happen?
A) Positively charged nucleus attracts electrons.
B) Coulomb’s law applies.
C) Accelerating electrons radiate energy.
D) Angular momentum is conserved.
Answer: C. Accelerating charges radiate energy, but the Bohr model accounts for quantum mechanics, where electrons in stable orbits do not radiate energy.
Photon Wavelengths: Which transition produces the photon with the longest wavelength?
A) n=2 to n=1
B) n=3 to n=1
C) n=3 to n=2
D) n=4 to n=1
E) n=4 to n=3
Answer: E. Longest wavelength corresponds to the smallest energy difference.







