Categorizing Matter by Composition
Matter, as we know, is anything that has mass and occupies space. It can be classified based on its physical state (solid, liquid, or gas) or its composition—the types of atoms or molecules that make it up. In this guide, we will focus on categorizing matter by its composition, building upon our earlier discussions.
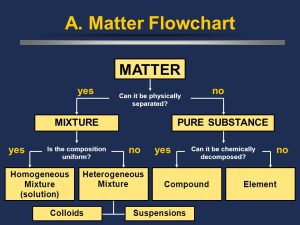
Pure Substances vs. Mixtures
When categorizing matter by composition, the first major distinction is between pure substances and mixtures:
Pure Substances are composed of a single type of atom or molecule. They have a consistent composition throughout. Examples of pure substances include elements like gold (Au) or compounds like water (H₂O).
Mixtures are made up of two or more substances that are physically combined but not chemically bonded. The composition of a mixture can vary from sample to sample.
Formula Units: Pure Substances vs. Mixtures
To better understand the difference between pure substances and mixtures, let’s look at formula units. A formula unit represents the lowest whole-number ratio of atoms in a compound. For example, a formula unit of sodium chloride (NaCl) is a 1:1 ratio of sodium to chloride ions.
Pure Substances have formula units of a single type, meaning every part of the substance is the same.
Mixtures contain formula units of different substances, which can vary in proportion.
Types of Mixtures
Mixtures can be further categorized into homogeneous and heterogeneous mixtures:
Homogeneous Mixtures have a uniform composition throughout, meaning you cannot see the individual components. The different substances are evenly distributed, making them look like a single substance.
Examples:
Salt Water (🌊): A mix of salt and water, which appears as one clear liquid.
Air (🛨️): A combination of different gases like nitrogen, oxygen, and carbon dioxide, but it looks the same everywhere.
Key Point: Homogeneous mixtures are often challenging to separate because the components are so evenly distributed. Imagine trying to separate soda into its individual ingredients—it would be incredibly difficult!
Heterogeneous Mixtures have visibly different components, and their composition is not uniform.
Examples:
Rocky Road Ice Cream (🍨): You can see the chocolate, nuts, and marshmallows all distinctly.
Salad (🥗): Each ingredient is visible and can be separated easily.
Key Point: Heterogeneous mixtures are easier to separate because their components are visibly distinct. You can easily take a salad apart, separating lettuce, tomatoes, cucumbers, etc.

Separating Mixtures
In chemistry, separating mixtures into their individual components is often crucial for experiments and analysis. There are several methods for separating mixtures, with two common techniques being distillation and filtration.
Distillation
Distillation is used to separate components in a liquid mixture by exploiting differences in their boiling points. The substance with the lowest boiling point will evaporate first, allowing it to be separated from the rest of the mixture.
Example: Consider a mixture of water and alcohol. Since alcohol has a lower boiling point than water, it will evaporate first. By collecting the vapor, you end up with a substance that is more concentrated in alcohol.
Filtration
Filtration is a technique used to separate heterogeneous mixtures by using a filter to trap solid particles while allowing liquids to pass through.
Example: Imagine filtering a mixture of sand, salt, and water. The sand (which is insoluble) will be caught by the filter, while the salt will dissolve in the water and pass through. To fully separate the salt from the water, you would need to evaporate the water, leaving behind the salt.
Note: Filtration only works for mixtures where there are insoluble solids. For complete separation, additional steps may be needed, such as evaporation.
Law of Definite Proportions
The law of definite proportions, also called the law of constant composition, is a fundamental principle that states that a chemical compound always contains the same elements in the same ratio by mass, regardless of the size or source of the sample.
Example: Water (H₂O) will always have two hydrogen atoms for every oxygen atom, no matter how much water you have or where it comes from.
Think of it like a recipe: the ingredients must always be combined in specific amounts to create the intended result. Similarly, compounds are formed by elements in fixed ratios, and if the proportions change, you have a different substance.
Empirical Formula
The empirical formula of a compound represents the simplest whole-number ratio of atoms in that compound. It shows the proportions of elements without specifying the exact number of atoms.
Example: The molecular formula for glucose is C₆H₁₂O₆, but its empirical formula is CH₂O. This means that the simplest ratio of elements in glucose is 1 carbon to 2 hydrogen to 1 oxygen.
Practice Problem: Empirical Formula
Suppose you are given a carbohydrate containing carbon, hydrogen, and oxygen with the following percent composition:
33.3% Carbon
7.4% Hydrogen
59.3% Oxygen (calculated by subtracting the other percentages from 100%)
Steps to Find the Empirical Formula:
Convert Percentages to Grams: Assume a 100g sample, which means the percentages are equal to grams.
33.3 g C, 7.4 g H, 59.3 g O
Convert Grams to Moles: Divide each mass by the molar mass of the element.
C: 33.3 g / 12.01 g/mol = 2.773 moles
H: 7.4 g / 1.008 g/mol = 7.34 moles
O: 59.3 g / 16.00 g/mol = 3.706 moles
Divide by Smallest Value: To simplify, divide all values by the smallest number of moles.
C: 2.773 / 2.773 = 1
H: 7.34 / 2.773 = 2.66
O: 3.706 / 2.773 = 1.33
Multiply to Get Whole Numbers: Since we have decimals, multiply by 3 to get whole numbers.
C: 1 × 3 = 3
H: 2.66 × 3 = 8
O: 1.33 × 3 = 4
Result: The empirical formula for this carbohydrate is C₃H₈O₄.
Thin-Layer Chromatography (TLC)
Chromatography is a powerful technique used to identify and compare mixtures by separating them based on their interaction with solids and liquids or differences in polarity (which will be further explored in later units).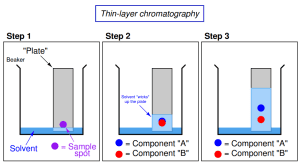
How Thin-Layer Chromatography Works
Imagine you have a piece of filter paper, and you draw a small dot of ink on it. The ink, being a mixture of various substances, can be separated using a process called thin-layer chromatography (TLC). Let’s go through the steps involved:
Applying the Sample: In the first step, a dot of the sample is placed near the bottom of the TLC plate (which is coated with a thin layer of silica or alumina). Most inks, for instance, contain a mixture of different pigments, making them perfect candidates for separation by TLC.
Solvent Movement: The TLC plate is then placed in a shallow container of solvent. The solvent slowly climbs up the TLC plate due to capillary action, bringing with it different components of the ink sample. As the solvent moves, it separates the sample into its individual substances based on how strongly each component interacts with the stationary and mobile phases.
Separation: The degree to which each substance moves depends on its chemical properties, especially polarity.
A Quick Rundown of Polarity
Polarity is a key concept in chemistry that helps explain how different substances interact. Simply put, a molecule is polar if it has a partial positive charge on one end and a partial negative charge on the other, resulting in an uneven distribution of electrical charge. A molecule that doesn’t have this uneven charge distribution is nonpolar.
A useful saying to remember is: “Like dissolves like.” This means that polar substances dissolve best in polar solvents, while nonpolar substances dissolve best in nonpolar solvents. For example, water (which is polar) and oil (which is nonpolar) don’t mix because they have different polarities.
TLC Phases: Stationary vs. Mobile
Stationary Phase: The TLC plate is coated with silica, a highly polar substance that acts as the stationary phase.
Mobile Phase: The solvent in the TLC chamber is the mobile phase. Depending on the solvent’s polarity, the components in the mixture will interact differently with both phases.
Generally, polar compounds are more attracted to the stationary phase (silica) and therefore do not move as far up the plate. Conversely, nonpolar compounds are less attracted to the stationary phase and are more easily carried by the mobile phase, allowing them to move farther up the plate.
Example: If a solvent used is nonpolar and there are two compounds—Compound A and Compound B. If Compound A travels farther up the TLC plate, that means it is less polar compared to Compound B and is more readily eluted by the nonpolar solvent. Compound B, being more polar, interacts more strongly with the polar silica, leading it to travel a shorter distance.
AP Practice Question – 2018 #2
This is an adapted question from the 2018 AP Chemistry Exam.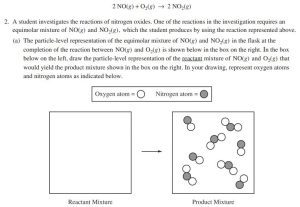
Imagine that you have a reactant mixture involving nitrogen monoxide (NO) and oxygen (O₂). You’re given information about the composition of the product mixture and asked to illustrate it.
Step 1: Identify the Reactants. We have NO and O₂ as reactants.
Step 2: Count the Atoms. There are 8 nitrogen atoms and 12 oxygen atoms in total.
Step 3: Drawing the Molecules. Since there are fewer nitrogen atoms, we’ll draw 8 NO molecules first. Then, we’ll draw the remaining O₂ molecules, with the leftover 4 oxygen atoms forming 2 O₂ molecules.

When answering questions like these, always use the symbols provided to ensure you’re following the instructions. The specific orientation of the molecules doesn’t matter as long as they are drawn correctly according to the given information.
AP Practice Question – 2017 #4
This question is taken from the 2017 AP Chemistry Exam and tests your understanding of thin-layer chromatography.
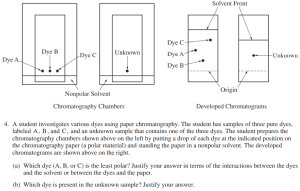
What We Know:
There are two TLC plates: one with labeled, known dots and one with an unknown dot.
The TLC plates are developed in a nonpolar solvent.
The starting point for each spot is provided, allowing comparison of how far each traveled.
To solve the question, consider how polarity impacts movement along the TLC plate. Since the solvent is nonpolar, the more nonpolar a compound is, the farther it will travel up the TLC plate. Conversely, polar compounds will stay closer to the starting point, attracted more to the polar stationary phase.
By comparing how far each component travels, we can infer the polarity of each and help identify unknown substances based on the distance traveled compared to known compounds.
Breaking Down Part (a):
Part (a) asks you to determine which of the known dyes (A, B, or C) is the least polar, and you must justify your answer using the interactions between the dyes and either the solvent or the TLC plate. To earn full points, it’s crucial to understand how polarity affects chromatography.
In this scenario, dye C travels the farthest, while dye B travels the least. Since the solvent is nonpolar, you can apply the rule of “like dissolves like” — meaning nonpolar substances are more soluble in nonpolar solvents and therefore travel farther. This implies that dye C is the least polar dye, as it is most attracted to the nonpolar solvent, while dye B, which is less mobile, is likely more polar.
Thus, the answer for part (a) could be phrased like this:
“Dye C is the least polar dye because it moved the farthest on the TLC plate. This is because nonpolar dyes are more attracted to the nonpolar solvent.”
Alternatively:
“Dye C is the least polar dye because it moved the farthest on the TLC plate. This suggests that nonpolar dyes interact less strongly with the polar stationary phase of the TLC plate.”
Both answers provide sufficient reasoning and demonstrate an understanding of the interaction between dye polarity, the solvent, and the stationary phase. Either response would receive full points.
Breaking Down Part (b):
Part (b) asks you to determine which dye is present in the unknown sample and to justify your answer. This part requires careful comparison between the TLC plates.
Notice that the solvent front of the TLC plate with the known dyes traveled farther than the solvent front for the unknown dye. This is important because to accurately compare them, the Rf (retention factor) of each dye must be calculated and compared proportionally to its own solvent front.
The unknown dye appears to have traveled roughly halfway between the origin and the solvent front. On the TLC plate with the known dyes, dye A also traveled halfway between its origin and the solvent front, suggesting that dye A matches the unknown.
According to scoring guidelines, your answer to part (b) could be written as follows:
“Dye A is present in the unknown sample. The unknown sample moved to a position that is midway between the origin and the solvent front, and dye A displayed similar behavior.”
Or:
“Dye A has a retention factor (Rf) close to 0.50 on the chromatogram with the known dyes, and the unknown dye also has an Rf close to 0.50.”
Retention Factor (Rf) Explained:
The retention factor, Rf, is a ratio that measures the distance traveled by a dye relative to the distance traveled by the solvent front. The Rf value helps compare how far each component moves under identical conditions. The formula for calculating Rf is:
Rf = (distance traveled by the component) / (distance traveled by the solvent)
The Rf value always falls between 0 and 1, providing a convenient way to compare different dyes even when the solvent fronts do not travel exactly the same distance. In this case, dye A’s Rf value matches that of the unknown dye, indicating a similarity between them.