Electrostatics: The Foundation of Electric Forces
Overview
Electricity is all around us—powering our homes, devices, and even natural phenomena like lightning. But what exactly is electricity? To understand it, we must first explore the concept of electrostatics, which forms the bedrock of electric forces and fields. In this unit, we’ll cover the basics of electric charge, Coulomb’s Law, and electric potential difference. Whether you’re revisiting these concepts or encountering them for the first time, this guide will help you build a solid foundation for understanding electricity.
Big Ideas in Electrostatics
Force Interactions: Forces characterize interactions between objects or systems.
Why does your hair stand on end after brushing it with a plastic comb?
Fields: Fields predict and describe interactions.
How does a charged rubber rod bend a stream of water?
Conservation: Conservation laws constrain interactions.
How are the kinematics of charged particles used in old televisions?
Real-World Implications:
Why can a bird land on a high-voltage wire and not be electrocuted?
How are maps of voltage similar to topographical maps?
Exam Impact
Unit 1 of electrostatics typically accounts for about 1/4 to 1/3 of the exam. Expect to spend around 40 class periods covering this material. The AP Classroom personal progress check includes approximately 35 multiple-choice questions and 1 free-response question to test your knowledge.
Section 1: Electric Charge & Coulomb’s Law
What is Charge? 🌩️
Charge is a fundamental property of subatomic particles. There are two types:
Positive Charge: Carried by protons.
Negative Charge: Carried by electrons.
The unit of charge is the Coulomb (C) for large amounts of charge, but for smaller quantities, we use the elementary charge (e):
Proton:
Electron:
Neutron:
In AP Physics C, charges are often treated as point charges (infinitely small objects with charge but no mass).
The Law of Electrostatics
Likes repel, opposites attract. Two positive charges repel each other, as do two negative charges. However, opposite charges (and) attract.
Using simulations like the PhET simulation, you can visualize how a charged balloon interacts with objects like a sweater or a wall.
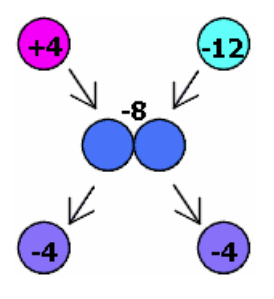
Conservation of Charge
The total charge in a system remains constant. For example, if a sphere with a charge of touches a sphere with a charge of , the charges redistribute equally, resulting in each sphere having a charge of.
Conductors vs. Insulators
Charge movement depends on the material:
Conductors: Allow charge to move freely (e.g., metals like copper).
Insulators: Restrict charge movement (e.g., rubber, plastic).
Conductors are often wrapped in insulating materials to prevent short circuits or electrocution, as seen in household wiring.
Charging and Discharging
Methods of Charging
Friction:
Rubbing two objects transfers electrons from one to the other.
The object losing electrons becomes positively charged, and the object gaining them becomes negatively charged.
Contact:
A charged object touches a neutral object, transferring its charge.
Induction:
A charged object brought near a neutral object polarizes it. If grounded, the neutral object becomes oppositely charged.
| Method | Initial Charge | Contact | Charge Movement | Final Charge |
|---|---|---|---|---|
| Friction/Rubbing | Neutral | Yes | Electrons move between objects | Two oppositely charged objects (+/-). |
| Contact | One charged, one neutral | Brief | Charges redistribute equally between objects | Both objects have the same charge. |
| Induction (Temporary) | Neutral | No | Opposite charges align; no net charge transfer | Object remains neutral. |
| Induction (Permanent) | Neutral | No | Grounding removes like charges; opposite charge remains | Object gains opposite charge. |
Coulomb’s Law: Calculating Electrostatic Force
Coulomb’s Law quantifies the force between two charges (and) separated by distance:
Where:
: Coulomb’s constant.
: Force (attraction or repulsion).
The force is repulsive if both charges have the same sign and attractive if they have opposite signs.
Applying Newton’s Laws
Electrostatic force is a force, so Newton’s three laws apply:
Equal and Opposite Forces:
Two charged objects exert equal and opposite forces on each other.
Acceleration:
A charged particle accelerates when acted upon by an electrostatic force.
Real-Life Examples
Charged Rod and Water:
A charged rod bends a stream of water due to attraction or repulsion of charges within the water molecules.
Household Wires:
Conductors carry current while insulators prevent electrical hazards.
Balloon and Hair:
Rubbing a balloon against hair transfers electrons, causing attraction.
Practice Questions
1. Charging by Induction
A positively charged rod is brought near a neutral electroscope. What happens?
Answer: The positive rod repels positive charges in the electroscope, causing the leaves to separate.
2. Direction of Forces
Two positive charges exert forces on a test charge placed between them. What is the net force direction?
Answer: The net force is directed away from both charges due to repulsion.
3. Comparing Electrostatic Forces
Rank the forces between pairs of charges:
| Pairs | Distance | Charge Magnitude |
| A & B | Long | Large |
| C & D | Short | Small |
| E & F | Medium | Medium |
Answer: Shorter distances result in stronger forces. Rankings: C = D > A = B > E = F.







