Electric Forces and Free-Body Diagrams
Electrostatic Force & Coulomb’s Law
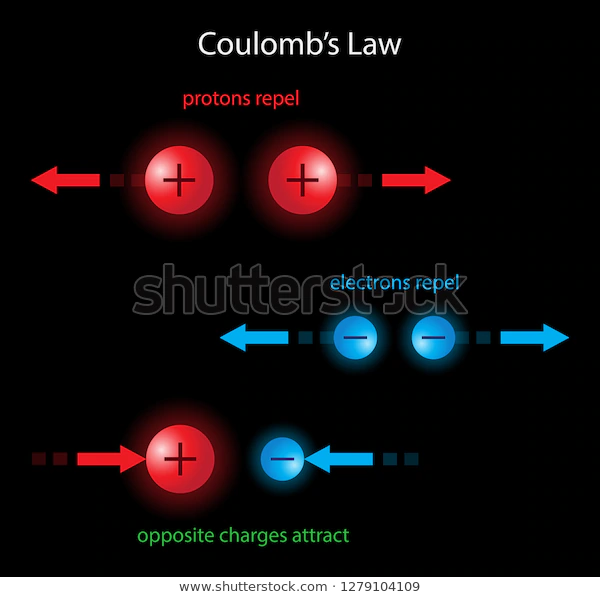
Coulomb’s Law describes the force of attraction (or repulsion) experienced between two charged point objects. Point charges simply mean that we can approximate the charges as acting from a single point. The equation for calculating electrostatic force is:
Where:
and : charges of the two objects
: distance between the charges
: Coulomb’s constant
Important Note: If and have the same sign, the force is positive, leading to repulsion. If they have opposite signs, is negative, indicating attraction.
Physics Reminder: Electrostatic force is a force! This means we need to apply Newton’s laws, especially the Third Law: For every action, there is an equal and opposite reaction.
Key Points about Electrostatic Force and Coulomb’s Law:
Electrostatic force arises between electrically charged particles.
Coulomb’s law quantifies the force, with magnitude depending on charge and distance.
The force can be attractive (opposite charges) or repulsive (same charges).
It applies to many phenomena, from atomic behavior to electric circuits.
Practice Questions
Question 1
Image from collegeboard.org
(a) What is the direction of the force on the test charge due to the two other charges?
(b) If is the magnitude of the force on the test charge due to only one of the particles, what is the net force acting on the test charge due to both of the charges?
Answers:
(a) The net force is directed towards the bottom left corner of the page. Since the test charge and both charges are positive, they repel. The test charge is repelled downwards and to the left.
(b) The test charge experiences two forces, both of magnitude , repelling it downwards and to the left. Using the Pythagorean theorem, the resulting net force can be determined:
Question 2
Image created by the author
Rank the magnitudes of the forces between the charge pairs.
Answer:
Each pair of charges must exert equal forces on each other (Newton’s Third Law).
Pairs & and & involve opposite charges (attraction), while & involve like charges (repulsion).
Force magnitude is proportional to charge product and inversely proportional to the square of the distance. Shorter distances (&) result in larger forces, even with smaller charge magnitudes.
Free-Body Diagrams (FBD)
Free-body diagrams (FBDs) are a powerful visualization tool for analyzing forces. They are especially useful in this unit for illustrating electric and gravitational forces, along with tension forces when applicable.
Drawing Free-Body Diagrams for Electric Forces
Identify the object of interest: Determine the object whose forces you want to analyze.
Determine force directions: Consider the magnitudes and directions of forces acting on the object.
Account for electric forces: Include forces such as Coulomb force and external forces like gravity.
Sketch the diagram: Represent the object as a point, draw force vectors as arrows, and label them appropriately.
Solve for net forces: Use vector addition and Newton’s laws to analyze the diagram.
Key Principles:
Electric force is directed with the electric field for a positive charge and opposite to it for a negative charge.
Newton’s Third Law applies: If charge 1 exerts a force on charge 2, charge 2 exerts an equal and opposite force on charge 1.
Example
If there is an electric field directed upward on the page, and an electron is placed at the center:
Electric Field Direction: Upward
Electric Force on Electron: Downward (opposite the field due to the electron’s negative charge)
Answer: The electric force will be towards the bottom of the page.
Summary
Electrostatic forces and Coulomb’s law are foundational to understanding electric interactions. Free-body diagrams help visualize and calculate these forces effectively. Mastery of these concepts is crucial for solving problems involving electric forces and dynamics.







