2.1 Thermodynamic Systems
Thermodynamics: An Overview 💤
Thermodynamics is the study of heat and energy. It involves concepts such as temperature, kinetic energy, heat, work, and energy conservation. Thermodynamics can be divided into two main branches:
Classical Thermodynamics 👓
Focuses on macroscopic phenomena, examining large systems and their equilibrium states.
Concerned with overall properties like pressure, volume, and temperature.
Provides a big-picture view, as commonly studied in Physics 1.
Statistical Thermodynamics
Analyzes microscopic processes at the atomic and molecular level.
Centers around the statistical behavior of particles, introducing concepts like entropy.
Explains the small-scale mechanisms driving the macroscopic behaviors observed in classical thermodynamics.
Efficiency: Bridging the Two
Efficiency unites classical and statistical thermodynamics, representing the ratio of input energy to output energy. Maximizing efficiency is a key concern in real-world engineering applications, as no machine is 100% efficient.
Key Differences Between Classical and Statistical Thermodynamics:
Approach: Classical focuses on macroscopic variables; statistical examines microscopic behavior.
Application: Classical applies broadly; statistical is more specific to particle interactions.
Concepts: Classical emphasizes properties like temperature; statistical delves into energy distributions among particles.
Thermodynamics: Systems and Boundaries
Thermodynamics examines interactions within a system and between a system and its surroundings.
Types of Systems:
Closed System: Exchanges energy (heat or work) but not matter.
Open System: Exchanges both energy and matter.
Isolated System: No exchange of energy or matter; total energy remains constant.
The boundary separates the system from its surroundings and defines the scope of study. Boundaries can be real (e.g., a container wall) or imaginary (e.g., a control surface).
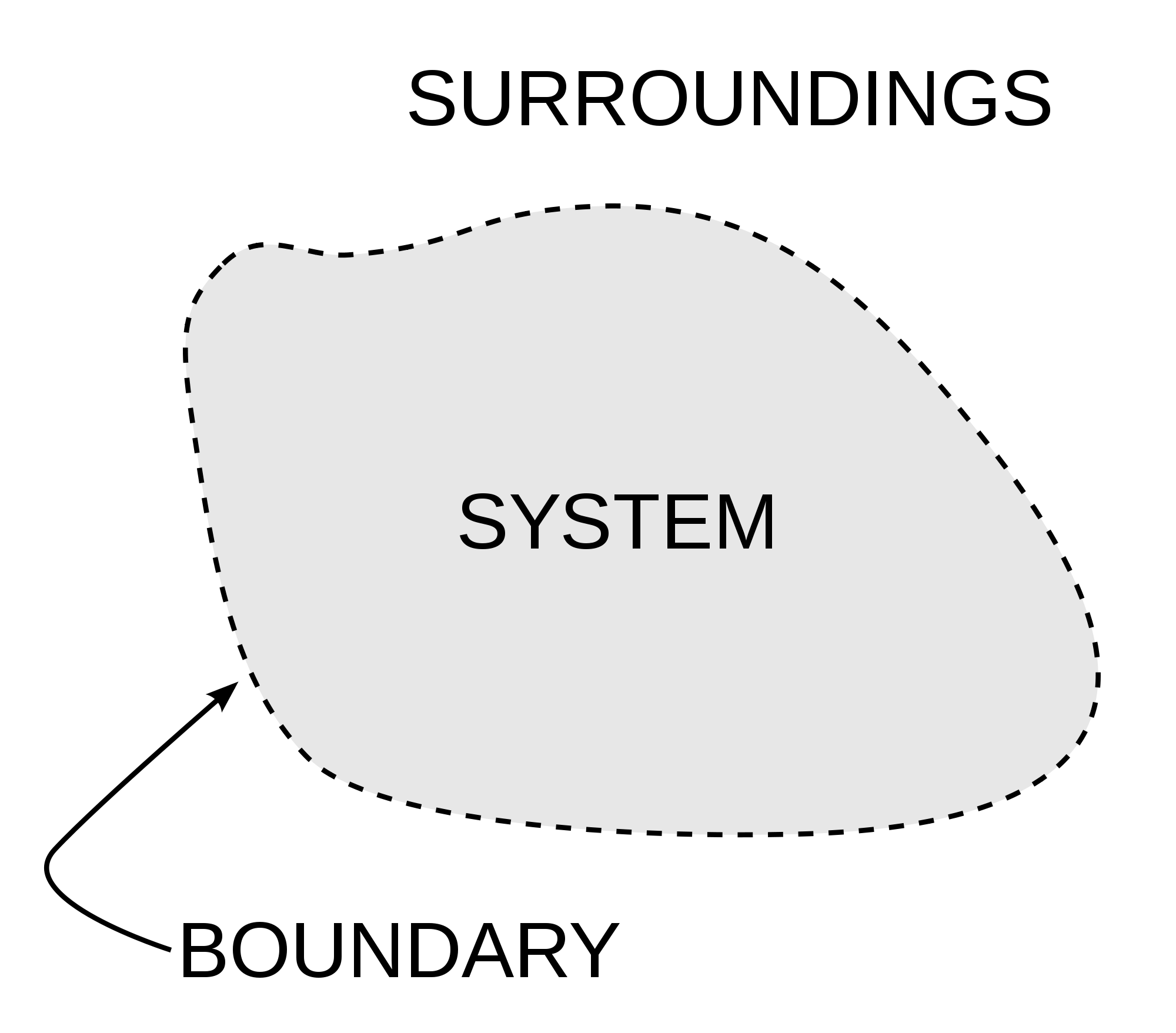
Object vs. System
Object: A defined physical entity, such as a piece of metal or a container of gas.
System: A collection of objects, defined by a boundary. Systems may include interacting particles and external exchanges of energy and matter.
In Physics 1, objects were often approximated as systems. In Physics 2, differentiating the two is essential for understanding thermodynamics. This distinction is particularly critical in Units 1 and 2.
Pro Tip: Pay attention to signs (positives and negatives) in calculations. Correctly assigning them ensures success in this section on the AP exam! 💕
Key Points:
Systems are influenced by interactions between constituent parts and external surroundings.
The laws of thermodynamics apply to systems, describing energy and matter exchanges.
Systems can be classified as open or closed based on their interaction with surroundings.
Example Problems
Problem 1:
A container is divided into two sections by a partition:
Initial State: Left contains 1 mole of gas A, right contains 1 mole of gas B.
Final State: Partition is removed; gases mix and reach equilibrium. Total pressure = 1 atm.
Tasks:
Draw the initial state showing the separated gases.
Draw the final state showing the mixed gases.
Problem 2:
Consider a solid object made of atoms:
Tasks:
Using a diagram, represent the interactions between the atoms.
Explain how these interactions determine properties like density, melting point, and strength.
Predict changes in interactions and properties if the solid is exposed to high temperatures.
In this unit, you will encounter pressure-volume (PV) diagrams, explore how machines like engines and refrigerators function, and apply mathematical models to analyze thermodynamic systems. Mastering the distinctions between objects and systems, along with accurate calculations, will set the foundation for success in understanding thermodynamics.







