8.3 Electric Force
Measuring Charge and Electric Force
What is Charge? 🔆
Charge is a fundamental property of matter, similar to mass, physical state, or density.
Neutral Atoms: Equal numbers of protons (positive charge) and electrons (negative charge) result in a net charge of 0.
Charged Atoms: Atoms become charged when electrons are added or removed:
Negative Charge: Gained electrons.
Positive Charge: Lost electrons.
Charge is entirely dependent on the movement of electrons.

How Do Things Become Charged? 🚗
There are two types of charge:
Positive Charge: Occurs when electrons are removed.
Negative Charge: Occurs when electrons are added.
Interactions:
Opposites Attract: Positive and negative charges attract each other.
Like Charges Repel: Two positive or two negative charges repel each other.
Dipoles: Even without changing the electron count, the arrangement of charges can create regions of partial charge. This separation of positive and negative charges within an object is referred to as a dipole.
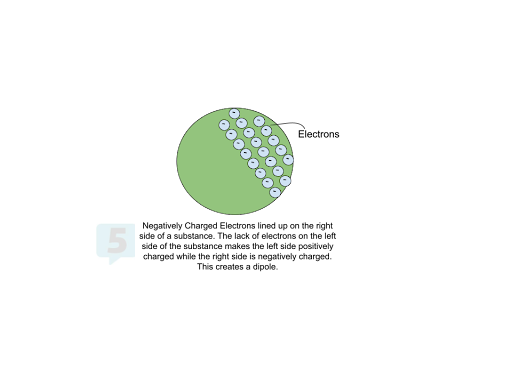
Measuring Charge 🎛
Unit of Measurement: Coulombs (C).
Elementary Charge (e): The charge of one electron: .
Charges in atoms are always integer multiples of :
Example: Removing 3 electrons gives a charge of .
Point Charges: Charged particles are often referred to as “point charges” when their charge is concentrated at a single point.

Electric Force 🔋
When two charges are near each other, they exert a force on one another that can be either:
Attractive: Opposite charges.
Repulsive: Like charges.
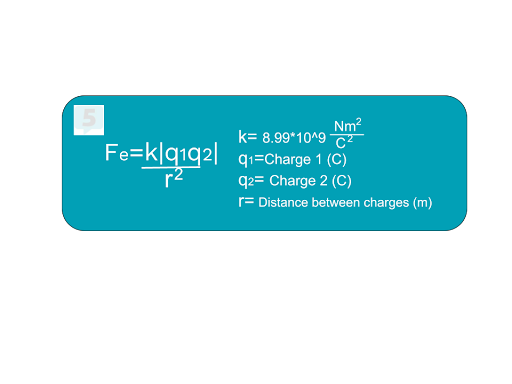
Coulomb’s Law:
The magnitude of the electric force between two point charges is given by:
Where:
: Electric force (N).
: Charges (C).
: Distance between charges (m).
: Coulomb’s constant .
Relationships:
Inverse Proportionality: Force decreases as distance increases.
Direct Proportionality: Force increases with greater charge magnitudes.
Coulomb’s Law Example 🔌
Problem:
Atom 1: Gains one extra electron.
Atom 2: Loses one electron.
Distance: 0.5 m.
Find the force between them.
Solution:
Identify variables:
, , .
Plug into Coulomb’s Law:
Solve:
Result: Each particle experiences an attractive force of .

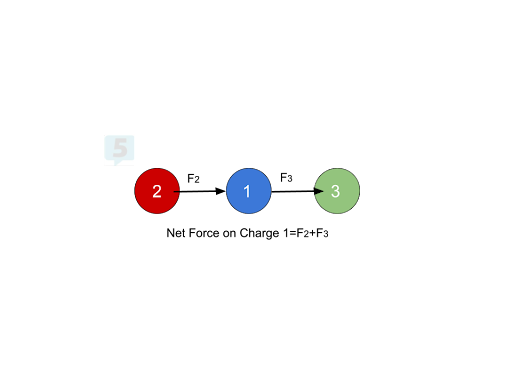
Superposition Principle ➕
When multiple electric forces act on a charge, their net force can be found by:
Adding Forces as Vectors: Treat forces as vectors to calculate the net force.
Example:
Charges: Atoms 1 and 2 are positive, Atom 3 is negative.
Forces:
Atom 2 repels Atom 1 to the right.
Atom 3 attracts Atom 1 to the right.
Net Force: Sum of forces from Atoms 2 and 3 results in a net force to the right.
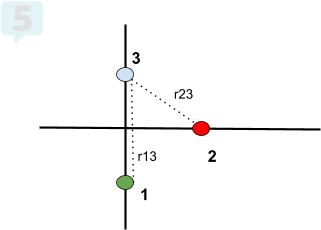
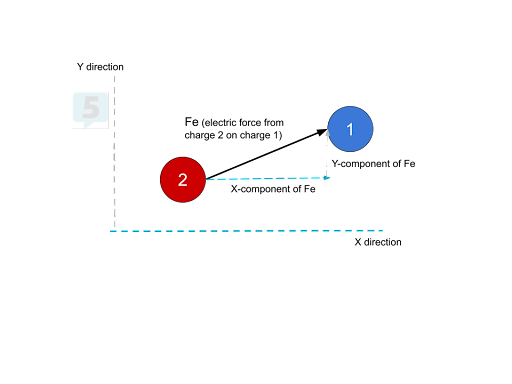
Net Forces at an Angle 💡
For forces acting at angles:
Break into Components:
Find x and y components of each force.
Find Net Force:
Add x components and y components separately.
Use the Pythagorean theorem to find the total force.
Determine Direction:
Use trigonometry to find the angle of the net force.
Practice Problems! ❔
Use Coulomb’s law and vector addition to solve these:
Electron Flow: 4.16 electrons move through a wire. How many Coulombs of charge were moved?
Attractive Force: A sock with of charge and a carpet with are 0.500 m apart. Find the electric force.
Net Force: Given:
at (0, -3).
at (3, 0).
at (0, 2).
Find the net force on .