Unit 9 Review: Applications of Thermodynamics in Chemistry
Welcome to the final unit of AP Chemistry! Throughout your journey, you’ve explored stoichiometry, chemical reactions, quantum mechanics, intermolecular forces, acids and bases, kinetics, equilibrium, and more. Now, it’s time to tackle the final challenge: thermodynamics. While thermodynamics was introduced in Unit 6 with thermochemistry, here we dive deeper into concepts like spontaneity, entropy, Gibbs Free Energy, and electrochemistry.
What is Thermodynamics?
In simple terms, thermodynamics is the study of energy changes in chemical reactions. While the prefix thermo- may make you think about temperature, it’s just one small part of the puzzle. The main focus is on spontaneity—determining whether a reaction will happen naturally or needs extra energy to proceed.
Imagine a ball rolling down a hill versus pushing it up—rolling down is spontaneous, but rolling it back up requires effort, making it nonspontaneous.
Key Concepts:
- System vs. Surroundings: The system is where the chemical reaction occurs, while the surroundings include everything outside the system. Together, they make up the universe.
This unit introduces two critical measures—entropy (S) and Gibbs Free Energy (ΔG)—to predict reaction spontaneity.
Major Topics in Unit 9
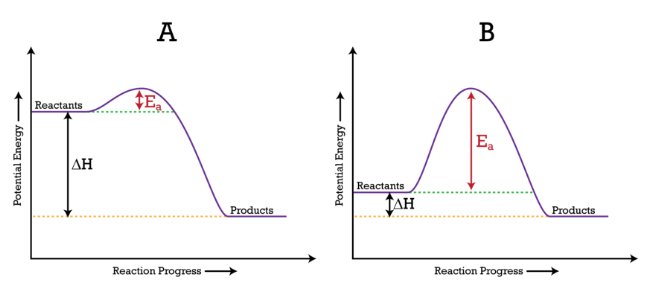
9.1-9.2: Entropy (ΔS)
Entropy quantifies the “disorder” in a system. Higher entropy implies more randomness, while lower entropy indicates more order. Reactions can either increase or decrease the system’s entropy:
- Increasing entropy (more disorder) is often associated with reactions that create gas from solids or liquids.
- Decreasing entropy (more order) might involve gas condensing to form a liquid or solid.
You’ll learn how to calculate entropy changes using values similar to enthalpies of formation and explore the laws of thermodynamics, which govern how energy moves through systems.
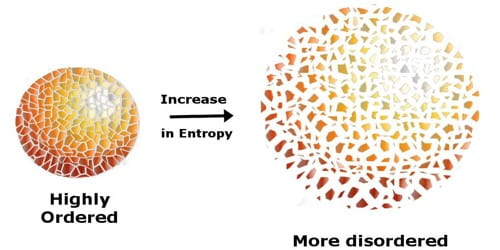
Image From AssignmentPoint
9.3-9.6: Gibbs Free Energy, Spontaneity, and Equilibrium
Together, enthalpy (ΔH) and entropy (ΔS) determine Gibbs Free Energy (ΔG), a measure of spontaneity:
- ΔG < 0: The reaction is spontaneous.
- ΔG > 0: The reaction is nonspontaneous.
- ΔG = 0: The system is at equilibrium.
Kinetic Control and Equilibrium
Even if a reaction is spontaneous, it may require a high activation energy, causing it to proceed slowly. This phenomenon is known as kinetic control.
Equilibrium and ΔG are also intertwined. At nonstandard conditions, ΔG indicates whether a reaction produces more reactants or products until equilibrium is reached.
Connection to Equilibrium Constant (K)
- ΔG° < 0 (spontaneous): K > 1 (product-favored).
- ΔG° > 0 (nonspontaneous): K < 1 (reactant-favored).
Image From Chemistry StackExchange
9.7-9.10: Electrochemistry
Electrochemistry studies redox reactions involving electron transfer. Here, you’ll explore:
- Galvanic (Voltaic) Cells: Spontaneous reactions producing electrical energy.
- Electrolytic Cells: Nonspontaneous reactions requiring an external electrical current.
Electrochemistry links cell potential (E°) to free energy changes (ΔG), and concepts of Q and K.
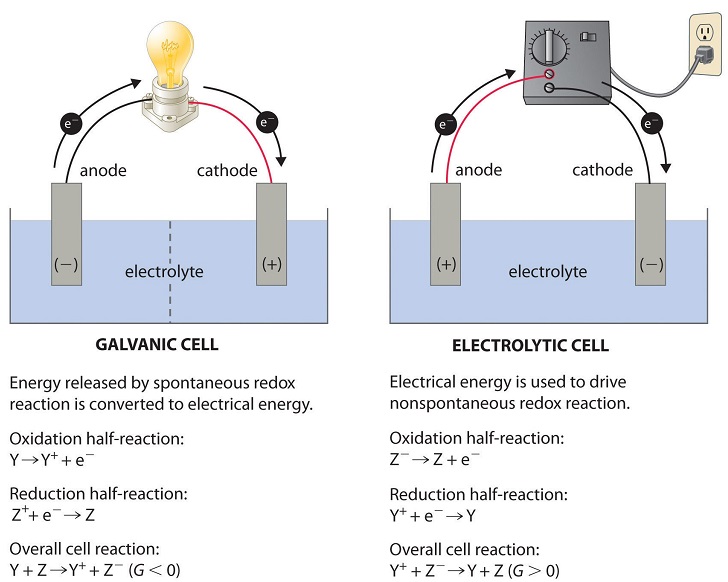
Image From LibreTexts/Electrochemistry/Electrolytic_Cells)
Practical Applications and Examples
Example: Predicting Reaction Spontaneity
Using the equation:
- A negative ΔG indicates a spontaneous reaction.
- For example, a reaction with ΔH < 0 (exothermic) and ΔS > 0 (increasing disorder) is always spontaneous.
Entropy in Everyday Life
Consider ice melting. As ice (solid water) transitions to liquid, its entropy increases since the molecules become more disordered.
Key Takeaways
- Thermodynamics predicts whether a reaction will occur spontaneously based on enthalpy, entropy, and Gibbs Free Energy.
- Kinetic control can slow spontaneous reactions if high activation energy is required.
- Electrochemistry connects redox reactions with energy transfer, vital for understanding batteries, corrosion, and more.
FAQs About Thermodynamics in Chemistry
Q: What is entropy?
A: Entropy (ΔS) measures the disorder in a system; higher values indicate greater randomness.
Q: How does Gibbs Free Energy relate to spontaneity?
A: If ΔG < 0, the reaction is spontaneous. If ΔG > 0, it’s nonspontaneous.
Q: What are galvanic cells?
A: Galvanic cells use spontaneous redox reactions to generate electrical energy.