Exploring the Molecular Structures of Acids and Bases: Strength and Stability Uncovered
Understanding the molecular structure of acids and bases provides a deeper look into their strengths. Why are some acids stronger than others, and what makes a base exceptionally weak or strong? By examining the Lewis structures and other molecular characteristics, we can uncover what influences the stability and reactivity of acids and bases. This guide breaks down how structural features connect with acid and base strength.
What Determines Acid and Base Strength?
Strong vs. Weak Acids and Bases
The strength of an acid or base largely depends on the stability of its conjugate. Here’s a quick refresher:
- Strong acids produce weak conjugate bases.
- Strong bases yield weak conjugate acids.
Conjugate Strength Relationship:
- A strong acid’s conjugate base is stable and non-reactive, while a weak acid’s conjugate base is much more reactive and less stable.
- Conversely, a strong base has a conjugate acid that is weak, while a weak base’s conjugate acid is stronger.
The Key Questions:
- For conjugate bases: Will this compound attract an H+ ion?
- For conjugate acids: Will this compound readily donate an H+ ion?
Connecting Structure to Strength
To evaluate acid and base strength through their molecular structure, we can consider several structural features and trends.
1. Bond Strength and Acidity
- Weak bonds to the acidic H atom typically indicate a stronger acid since these bonds are more easily broken.
- For example, consider the halogenic hydrides: HF, HCl, HBr, and HI. As we move down the periodic table, the size of the halogen increases, weakening the H-X bond. As a result, HI is a stronger acid than HF because the H-I bond breaks more easily.
Trend Reminder:
- Acid strength increases across a period (left to right) and down a group in the periodic table.
- Strong acids form very stable conjugate bases. For example, the conjugate base of HCl is Cl-, which is stable and not reactive, making HCl a strong acid.
Image From FHS AP Chemistry
2. Oxyacids and Polarity
For oxyacids (acids that contain oxygen), their strength often depends on the bond polarity between the acidic O-H group.
Example:
- Consider a generic oxyacid represented as HO-Z, where Z is the rest of the molecule. The easier it is for the O-H bond to break, the stronger the acid.
- If Z is highly electronegative or has a high oxidation state, it draws electron density away from the O-H bond, making it easier to break. This results in a stronger acid.
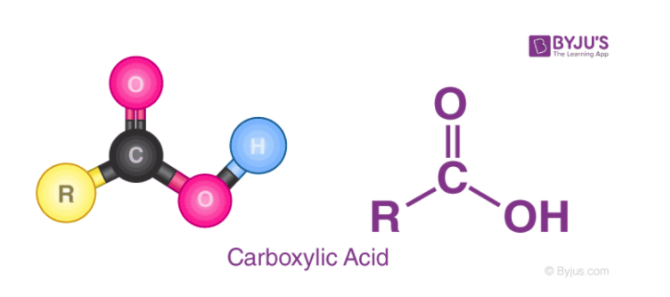
Carboxylic Acids as a Case Study:
- Carboxylic acids (e.g., CH3COOH) are common examples of weak acids. The presence of the COOH group influences their behavior.
- Due to the low electronegativity of carbon in the structure, the bond to the acidic hydrogen is less polar and less readily dissociates, making acetic acid (CH3COOH) weaker.
3. Electronegativity and Inductive Effects
- Electronegative elements near the acidic hydrogen can pull electron density through inductive effects, increasing acidity. For instance, more electronegative groups attached to a molecule’s structure enhance its acid strength by stabilizing the conjugate base.
Visual Representations of Molecular Structure
Let’s break down a few images to solidify our understanding.
Halogenic Hydrides (HF, HCl, HBr, HI) – Depicting how acid strength increases as the bond between hydrogen and the halogen weakens down the group.
Oxyacid Structure with HO-Z – Highlighting how the strength depends on the nature of Z.
Carboxylic Acid Structure (CH3COOH) – Explaining why this common acid is relatively weak due to low polarity and weaker bonds.
Key Takeaways
- Bond Strength Matters: Weaker bonds with acidic H indicate stronger acids.
- Electronegativity and Polarity Influence Strength: High electronegativity and highly polarizable bonds make it easier for acids to donate protons.
- Conjugate Stability: Strong acids form weak conjugate bases, while strong bases form weak conjugate acids.
- Oxyacids and Functional Groups: Acid strength in oxyacids depends on the electronegativity of atoms bonded near the acidic group.
Image From Byju’s