What Are Acids and Bases? A Comprehensive Guide
The terms “acid” and “base” often evoke images of sour lemons or soapy cleaners. While the general public might have a basic grasp of what acids are, the concept of bases tends to be less well-known. However, understanding acids and bases is crucial in chemistry as they play a pivotal role in countless reactions and processes, from digestion in our stomachs to industrial cleaning solutions.
Let’s explore what acids and bases are, how they work, and why they matter!
What Are Acids?
Definition and Characteristics of Acids
In chemistry, acids are substances that have a pH of less than 7 when dissolved in water. They have distinct properties such as:
- Sour taste (think of lemon juice).
- The ability to react with bases to form salts and water.
- The capacity to donate protons (H⁺) to other substances, making them proton donors.
Example: When hydrochloric acid (HCl) dissolves in water, it releases H⁺ ions, which can react with bases.
Types of Acids
- Mineral Acids: These are inorganic acids, such as hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
- Organic Acids: These include compounds like acetic acid (CH₃COOH) found in vinegar.
Strength of Acids
- Strong Acids: Fully dissociate in water, releasing all their hydrogen ions. Examples include HCl and H₂SO₄.
- Weak Acids: Partially dissociate in water. Acetic acid is a common example.
The strength of an acid is denoted by its acidity, which measures the concentration of H⁺ ions in solution. Strong acids have a higher acidity and a greater capacity to donate protons than weak acids.
What Are Bases?
Definition and Characteristics of Bases
Bases are substances that have a pH greater than 7. They are known for their:
- Bitter taste and slippery feel (like soap).
- Ability to react with acids to form salts and water through a process called neutralization.
- Capability to accept protons (H⁺), making them proton acceptors.
Example: Sodium hydroxide (NaOH), commonly known as lye, is a strong base that dissociates in water to produce OH⁻ ions.
Types of Bases
- Inorganic Bases: Examples include sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)₂).
- Organic Bases: Examples include compounds such as amines.
Strength of Bases
- Strong Bases: Completely dissociate in water to release OH⁻ ions. Examples include NaOH and KOH.
- Weak Bases: Partially dissociate in water. Ammonia (NH₃) is a well-known weak base.
The strength of a base is measured by its basicity, which indicates the concentration of OH⁻ ions in solution. Strong bases have a higher basicity, meaning they accept more protons than weak bases.
The pH Scale: Acids, Bases, and Neutral Solutions
The pH scale measures how acidic or basic a solution is, ranging from 0 (strongly acidic) to 14 (strongly basic):
- Acidic solutions: pH < 7
- Neutral solutions: pH = 7 (equal concentration of H⁺ and OH⁻ ions)
- Basic (alkaline) solutions: pH > 7
Why Do Acids and Bases Matter?
Acids and bases are fundamental to biochemical reactions, industrial processes, food production, and medicine:
- Stomach acid (HCl) helps in digestion.
- Bases are used in cleaning products, fertilizers, and pharmaceuticals.
- The interaction between acids and bases leads to the formation of salts and water in neutralization reactions.
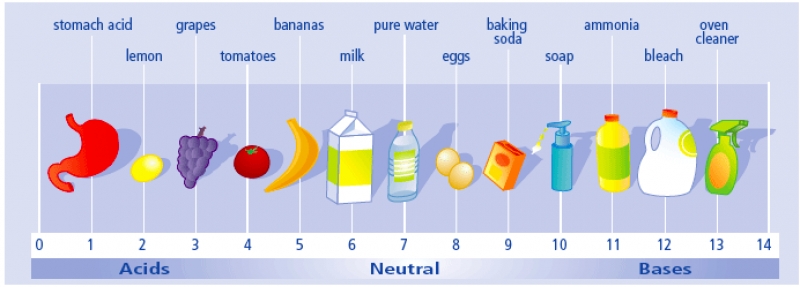
The pH scale is something you will be very familiar with by the time you finish Unit 8.
Introduction to Acid-Base Chemistry in Unit 8
Unit 8 dives deep into acid-base chemistry, focusing on how free protons (H⁺ ions) move and interact when dissolved in a solution:
- pH and pOH calculations: Measuring the concentration of H⁺ and OH⁻ ions.
- Buffers: Mixtures that maintain a stable pH by neutralizing small amounts of added acid or base.
- Titrations: A method for determining the concentration of an acid or base using a known volume of a titrant.
Why It Matters
Acid-base reactions are the basis for countless biological processes and chemical reactions, making them a cornerstone of chemistry and life itself!
Learning Summary
- Acids are proton donors (pH < 7) with a sour taste.
- Bases are proton acceptors (pH > 7) with a bitter taste.
- The pH scale helps measure the acidity or basicity of a solution.
- Acid-base chemistry plays a vital role in everyday life, from digestion to cleaning.







