7.14 Free Energy of Dissolution: Thermodynamics and Solubility
Bringing Together Thermodynamics and Solubility in Chemistry
In this final segment of Unit 7, we’ll explore the connection between solubility and thermodynamics. Thermodynamics studies energy transfers during chemical reactions and helps explain why certain substances dissolve while others don’t. We’ll touch on key concepts like enthalpy, entropy, and Gibbs Free Energy (ΔG) and discuss how they relate to the dissolution of soluble and sparingly soluble compounds.
Quick Crash Course: Thermodynamic Concepts
To understand how solubility relates to energy changes, let’s briefly cover key thermodynamic concepts.
1. Entropy (S): A Measure of Disorder
Entropy describes the level of disorder or randomness in a system. The greater the number of possible arrangements, the higher the entropy.
- Example: Imagine a messy room versus a tidy one. It’s easy to create disorder (high entropy) but requires effort (energy) to bring order.
- In chemistry, states of matter illustrate entropy: solids are highly ordered (low entropy), liquids are less ordered, and gases are highly disordered (high entropy).
Entropy Changes in Phase Transitions:
- Melting and evaporation increase entropy as the structure becomes less ordered.
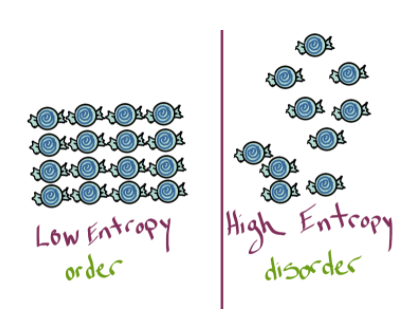
Image From InfluxData
2. Gibbs Free Energy (ΔG): Predicting Thermodynamic Favorability
Gibbs Free Energy (ΔG) is a measure that combines enthalpy (ΔH) and entropy (ΔS) changes to determine if a reaction is spontaneous (thermodynamically favorable).
- Formula for ΔG:
- ΔG < 0: The reaction is spontaneous (favorable).
- ΔG > 0: The reaction is nonspontaneous (unfavorable).
- ΔG relates to the equilibrium constant (K). When ΔG < 0, K > 1, indicating that the reaction favors the formation of products.
How ΔG Relates to Dissolving Substances
When a substance dissolves:
- ΔH (Enthalpy Change): Energy is either absorbed or released as bonds break and form.
- ΔS (Entropy Change): The disorder changes as the solid dissolves into ions or molecules in solution.
Dissolution: Breaking and Forming Bonds
When a solute dissolves, energy changes involve breaking and forming bonds:
- Solute-Solute Interactions: Breaking the crystal lattice structure requires energy (endothermic).
- Solvent-Solvent Interactions: Breaking hydrogen bonds or other interactions within the solvent also requires energy.
- Solute-Solvent Interactions: New bonds form between the solute and solvent (exothermic).
Key Idea: The overall ΔG determines if dissolution is thermodynamically favorable:
- ΔG < 0: Dissolution is spontaneous.
- ΔG > 0: Dissolution is not spontaneous (low Ksp).
Example: Thermodynamic Favorability of Dissolution
Consider the dissolution of a salt like CaCO₃:
- ΔH: Breaking ionic bonds (lattice energy) in CaCO₃ and hydrogen bonds in water requires energy.
- ΔS: Dissolving increases disorder as ions disperse in solution.
- If ΔH is significantly positive (endothermic), ΔS must be large enough to make ΔG negative for spontaneous dissolution.
Summary Table: Dissolution Thermodynamics
| Parameter | Description | Example in Dissolution |
|---|---|---|
| ΔH (Enthalpy) | Energy change when bonds break/form | Breaking ionic lattice (endothermic), forming bonds (exothermic) |
| ΔS (Entropy) | Measure of disorder/change in randomness | Ions dispersing in solution (increased disorder) |
| ΔG (Gibbs Free Energy) | Determines spontaneity (favorable/unfavorable) | Negative ΔG = Favorable dissolution; Positive ΔG = Unfavorable dissolution |
Practical Insights on Gibbs Free Energy and Solubility
- Highly Soluble Compounds: Have negative ΔG due to favorable enthalpy and/or entropy changes, resulting in high Ksp values.
- Sparingly Soluble Compounds: Positive ΔG due to unfavorable energy balance, resulting in low Ksp values.
- Temperature Dependence: The term -TΔS shows that solubility can be temperature-dependent, especially for processes with significant entropy changes.
Learning Summary ✏️
- ΔH and ΔS play critical roles in determining ΔG and whether a compound will dissolve spontaneously.
- Thermodynamic favorability relates to solubility via ΔG and Ksp values.
- Both enthalpy and entropy changes must be considered for a holistic view of solubility and dissolution.