7.13 pH and Solubility: A Deep Dive into Chemical Equilibrium
Understanding pH and Solubility in Chemistry
The concepts of pH and solubility are deeply interconnected in chemical equilibrium, especially when linked with Le Chatelier’s Principle and the common ion effect. pH measures the concentration of hydrogen ions (H⁺) and, inversely, hydroxide ions (OH⁻) in a solution, impacting solubility equilibria significantly. By understanding these relationships, we can predict how the solubility of various compounds is influenced by acidic or basic conditions.
How pH Affects Solubility in Acidic Solutions
The pH of a solution can strongly impact the solubility of compounds, particularly those that dissociate into a conjugate base of a weak acid. Here’s a quick refresher: the conjugate base forms when an acid loses a proton (H⁺), while the conjugate acid forms when a base gains a proton.
Image From Purdue University
Why pH Matters for Conjugate Bases
- Conjugate bases of weak acids are basic themselves, unlike the conjugate bases of strong acids (e.g., Cl⁻, Br⁻, ClO₄⁻), which do not significantly affect solubility.
- Consider the reaction of a basic conjugate base A⁻ with water:
- When the solution is acidic (high H⁺ concentration), H⁺ ions react with A⁻ to form HA, thereby decreasing [A⁻] and shifting the equilibrium to produce more HA. This decreases Q (reaction quotient), making the reaction shift right according to Le Chatelier’s Principle, increasing solubility.
Example Problem: Effect of pH on Solubility
Consider the solubility equilibrium:
In an acidic solution, the high concentration of H⁺ ions reacts with OH⁻ to form H₂O, decreasing [OH⁻]. According to Le Chatelier’s Principle, this decrease causes the equilibrium to shift right, increasing the solubility of Fe(OH)₃.
Solubility in Basic Solutions
The impact of a basic solution on solubility is essentially the reverse of what occurs in acidic conditions:
- Basic solutions increase [OH⁻]. Compounds that form conjugate acids (e.g., NH₄⁺) become more soluble because the OH⁻ ions react with NH₄⁺ to reduce [NH₄⁺], decreasing Q and pushing the equilibrium towards more dissolution.
- Conversely, basic compounds like strong bases (e.g., Ba(OH)₂) become less soluble due to the common ion effect from the already high [OH⁻] concentration.
- For weak conjugate bases (e.g., CH₃COO⁻), the equilibrium: CH₃COO⁻ + H₂O⇋CH₃COOH + OH⁻ shifts left due to increased OH⁻, reducing solubility.
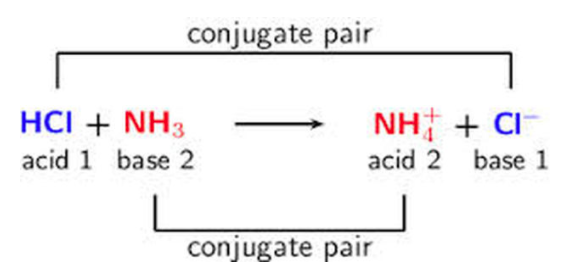
Image From Acids and Bases 101
pH-Neutral Compounds and Solubility
pH-neutral compounds (e.g., NaCl) dissociate into ions that do not influence acidity or basicity (e.g., Na⁺ and Cl⁻, which are conjugates of strong bases and acids). As such, pH has no direct impact on their solubility unless a common ion is present in the solution.
Key Takeaways on pH and Solubility
- Acidic solutions increase the solubility of compounds that dissociate into basic anions.
- Basic solutions increase the solubility of compounds that dissociate into acidic cations.
- pH-neutral compounds are largely unaffected by pH, except for the common ion effect.
- Le Chatelier’s Principle explains the shifts in equilibrium due to changes in pH.
Practice Problem Recap
Problem: Consider the equilibrium:
How does solubility change in an acidic solution?
Solution: In an acidic solution, H⁺ ions react with OH⁻ to form H₂O, reducing [OH⁻] and shifting the equilibrium right, increasing solubility.
FAQs on pH and Solubility
Q: Why are compounds that dissociate into conjugate bases of weak acids more soluble in acidic solutions?
A: The H⁺ ions react with the basic conjugate base, reducing its concentration and shifting the equilibrium towards more dissolution.
Q: How does pH affect strong bases like NaOH?
A: Strong bases become less soluble in basic solutions due to the common ion effect from already high [OH⁻].







