7.11 Introduction to Solubility Equilibria
What Is Solubility Equilibria?
Solubility is a topic we explore extensively in AP Chemistry, focusing on whether a solute can dissolve in a solvent—usually water. By incorporating our understanding of equilibrium, we can dive deeper into the dissolution of compounds, even those traditionally labeled as “insoluble.”
In reality, every compound dissolves to some extent, even if it’s only a tiny amount. For example, NaCl dissolves readily, while a compound like PbI₂ is labeled as insoluble due to its significantly smaller solubility. But even PbI₂ dissolves slightly, as demonstrated by the equilibrium reaction:
PbI₂(s) ⇌ Pb²⁺(aq) + 2I⁻(aq)
While we might think of this reaction as practically non-existent due to the low solubility of PbI₂, it still has a small Ksp (solubility product constant) value of 4.41 x 10⁻⁹. When working with solubility equilibria, Ksp tells us how much of a compound dissolves and represents the product of the concentrations of the resulting ions, each raised to the power of their coefficients. Solids are not included in these equilibrium calculations.
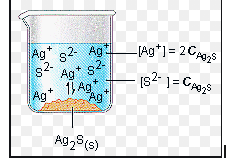
Image From Purdue University
The Importance of Ksp Values
For highly soluble compounds, such as NaCl or KOH, Ksp values are typically much greater than 1. This indicates that these compounds dissolve almost completely, often leading us to treat the dissolution process as irreversible. On the other hand, a very small Ksp value, like in the case of PbI₂, indicates a limited extent of dissolution.
Calculating Ksp: A Step-by-Step Example
Problem: Calculate the Ksp of PbCrO₄ given its solubility is 4.5 x 10⁻⁵ g/L.
Write the Dissolution Reaction:
PbCrO₄(s) ⇌ Pb²⁺(aq) + CrO₄²⁻(aq)Convert Solubility to Molarity:
Calculate Ksp:
Note: Remember to adjust equilibrium concentrations for coefficients if present!
Calculating Molar Solubility
Molar solubility calculations involve finding the equilibrium concentrations of ions in a saturated solution using Ksp. The process often involves setting up an ICE Table (Initial, Change, Equilibrium). Here’s a practice problem:
Problem: Calculate the molar solubility of CaCO₃ with a Ksp value of 3.36 x 10⁻⁹.
Write the Dissolution Reaction:
CaCO₃(s) ⇌ Ca²⁺(aq) + CO₃²⁻(aq)Set Up an ICE Table:
Reaction CaCO₃ Ca²⁺ CO₃²⁻ Initial — 0.00 M 0.00 M Change — +x +x Equilibrium — x x Calculate Ksp:
Given Ksp = 3.36 x 10⁻⁹:
Relating Qsp and Ksp
Just as we use the reaction quotient Q to determine shifts in chemical equilibrium, Qsp (reaction quotient for solubility) helps predict the formation or dissolution of precipitates in a solution:
- Qsp > Ksp: The reaction will shift to form more solid precipitate (reactants).
- Qsp < Ksp: The reaction will shift to produce more ions in solution (products).
- Qsp = Ksp: The system is at equilibrium; no net change occurs.
Key Takeaways
- Ksp indicates the extent of a compound’s solubility.
- Qsp helps predict the direction of a solubility reaction based on current ion concentrations.
- For highly soluble substances, Ksp values are large, while “insoluble” compounds have small Ksp values.
- ICE Tables are invaluable for calculating molar solubility and understanding solubility equilibria dynamics.







