7.4 Calculating the Equilibrium Constant
Understanding Equilibrium Constants
Now that we’ve covered what Kc (equilibrium constant using concentration) and Kp (equilibrium constant using partial pressure) represent, let’s dive into how to actually calculate these values. Calculating equilibrium constants allows chemists to determine the extent of a reaction and understand whether a reaction is product-favored or reactant-favored at equilibrium. Before we get started, remember that K is a unitless value, and only gases and aqueous solutions are included in the equilibrium expression.
Formulas for Kc and Kp
Kc and Kp both follow the general format:
Kc: Uses molar concentrations (mol/L)
Kc = [products] / [reactants], where concentrations are raised to their stoichiometric coefficients.Kp: Uses partial pressures (in atm)
Kp = (partial pressure of products) / (partial pressure of reactants), also raised to stoichiometric coefficients.
For example, consider the reversible reaction A + B ⇌ C + D:
- Kc = [C][D] / [A][B]
- Kp = P(C) * P(D) / P(A) * P(B)
It’s important to remember that solids and liquids are not included in equilibrium expressions.
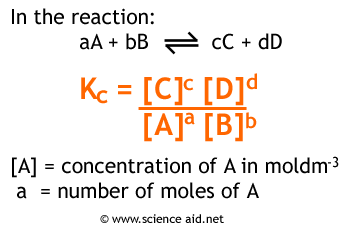
Image Courtesy of ScienceAid

Image Courtesy of Quora
Calculating Kc: An Example
Problem: Calculate Kc for the following system if 0.1908 moles of CO₂, 0.0908 moles of H₂, 0.0092 moles of CO, and 0.0092 moles of H₂O vapor are present in a 2.00 L reaction vessel at equilibrium.
Step-by-Step Solution:
Write the equilibrium expression:
For the reaction, the expression for Kc is:
Kc = [CO][H₂O] / [CO₂][H₂]Find equilibrium concentrations:
Convert moles to molarity (M) by dividing by the volume (2.00 L):- [CO] = 0.0092 moles / 2.00 L = 0.0046 M
- [H₂O] = 0.0092 moles / 2.00 L = 0.0046 M
- [CO₂] = 0.1908 moles / 2.00 L = 0.0954 M
- [H₂] = 0.0908 moles / 2.00 L = 0.0454 M
Calculate Kc:
Plug these values into the Kc expression:
Kc = [0.0046][0.0046] / [0.0954][0.0454] = 4.9 x 10⁻³
Calculating Kp: An Example
Problem: Calculate Kp for the reaction 2N₂O₅ (g) ⇌ O₂ (g) + 4NO₂ (g), given the following partial pressures:
- P(N₂O₅) = 2.00 atm
- P(O₂) = 0.296 atm
- P(NO₂) = 1.70 atm
Step-by-Step Solution:
Write the Kp expression:
Kp = P(O₂) * [P(NO₂)]⁴ / [P(N₂O₅)]²Plug in the values:
Kp = (0.296) * (1.70)⁴ / (2.00)²
Kp = (0.296) * (8.3521) / 4.00
Kp ≈ 0.618
Why the Equilibrium Constant Formula Works
The equilibrium constant formula, whether for Kc or Kp, compares the relative amounts of products and reactants at equilibrium:
- K > 1 indicates a product-favored reaction, meaning more products are formed at equilibrium.
- K < 1 indicates a reactant-favored reaction, meaning more reactants remain at equilibrium.
This relationship arises from the ratio of product to reactant concentrations at equilibrium. For instance, a large K value suggests that equilibrium heavily favors product formation, whereas a small K suggests that the reaction doesn’t proceed far forward.
Tips for Calculating Equilibrium Constants
- Use Equilibrium Values: Ensure you’re plugging in values at equilibrium; otherwise, you’ll be calculating Q (reaction quotient) instead of K.
- Correct Units: Convert all values to the proper units (e.g., mol/L for Kc).
- Account for Stoichiometry: Raise concentrations or pressures to their respective coefficients from the balanced equation.
- Watch for Exclusions: Solids and pure liquids are not included in equilibrium expressions.
- Be Prepared for Unit Conversions: Some problems may require converting between grams, moles, molarity, or partial pressures.
Practice Problem
AP Chemistry 2017 Question:
Given the reaction:
N₂(g) + O₂(g) ⇌ 2NO(g)
(a) Write the expression for the equilibrium constant, Kp, for the forward reaction.
Solution:
The Kp expression is:
Kp = (P(NO))² / (P(N₂) * P(O₂))
Final Thoughts
Understanding how to calculate equilibrium constants provides deep insights into chemical reactions and their tendencies. Keep practicing with different scenarios to solidify your understanding of Kc and Kp!
Key Takeaways
- Kc uses molar concentrations, while Kp uses partial pressures.
- Calculating K involves using equilibrium values only.
- A large K value means a product-favored reaction, while a small K indicates a reactant-favored reaction.
- Stoichiometric coefficients must be considered in the equilibrium expression.