AP Chemistry Unit 5: Mastering Chemical Kinetics
Introduction
Welcome to Unit 5 of AP Chemistry, where we shift from understanding what happens during chemical reactions to exploring how fast reactions occur and the factors influencing these rates. Known as Kinetics, this unit dives into the detailed study of reaction rates, how to control and measure them, and the underlying molecular collisions driving chemical changes.
This unit carries significant weight in the AP Chemistry exam, making up 7-9% of the content. Ready to master Kinetics? Let’s dive in!
Big Idea Questions
- Why are some reactions faster than others?
- How long does it take for a marble statue to erode? 🗿
- How does a sports drink alleviate headaches? 🤕
- Why does bread rise? 🍞
5.1 Reaction Rates
Reaction rate is a measure of how quickly a chemical reaction occurs. It is determined by the change in concentration of a reactant or product over time. The rate can be mathematically expressed as:
- Rate = -Δ[Reactant]/t or Rate = Δ[Product]/t, where
Δ represents the change, and t is time.
Units for reaction rate are typically mol/Ls or Ms⁻¹.
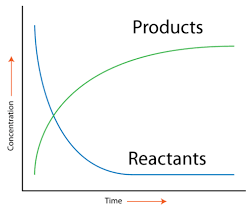
Image Courtesy of CK-12
Graphically, the rate of a reaction is represented by the slope of the line between two points on a concentration vs. time graph. A steeper slope indicates a faster reaction.
5.2 Introduction to Rate Law
A rate law quantifies the relationship between the reaction rate and the concentration of reactants. It takes the form:
R=k[A]n[B]m
- R = Rate of the reaction
- k = Rate constant (temperature-specific)
- [A] and [B] = Concentrations of reactants
- n and m = Reaction orders (experimentally determined)
The rate constant (k) changes with temperature, affecting the reaction’s speed.
5.3 Concentration Changes Over Time
To understand how reactant concentrations change over time, we use integrated rate laws:
- Differential rate law describes how reaction rate changes with concentration.
- Integrated rate law shows how concentration changes over time.
Key Graphs:
- Zeroth-order reactions: [A] vs. time (linear)
- First-order reactions: ln[A] vs. time (linear)
- Second-order reactions: 1/[A] vs. time (linear)
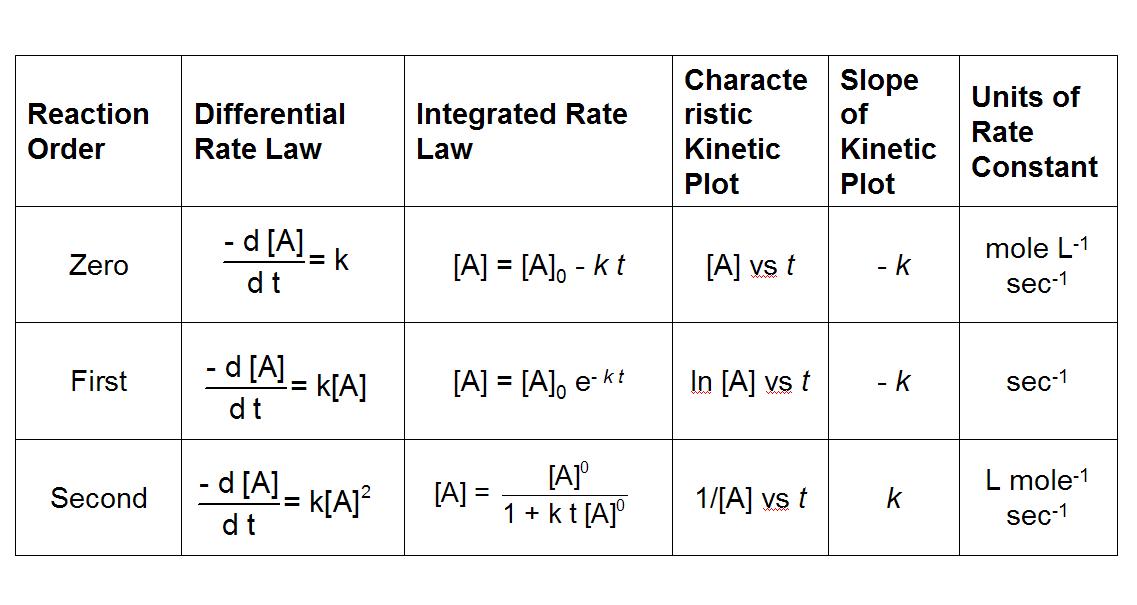
Image Courtesy of Chem FSU
5.4 Elementary Reactions and Rate Laws
Elementary reactions are single-step processes that make up more complex reaction mechanisms. Importantly, reaction orders cannot be deduced solely from stoichiometric coefficients unless verified experimentally.
To determine rate laws, experiments are conducted by varying reactant concentrations and observing rate changes.
5.5 Collision Model
The collision model explains why reactions occur. For a reaction to proceed:
- Reactant molecules must collide.
- Collisions must occur with sufficient energy (overcome activation energy) and correct orientation.
The frequency of effective collisions determines the reaction rate.
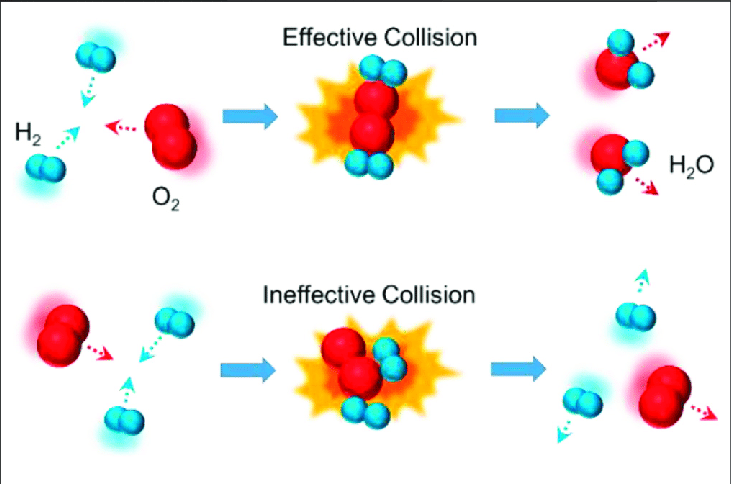
Image Courtesy of Research Gate
5.6 Reaction Energy Profile
Activation energy (Ea) is the minimum energy required for a reaction to proceed. A reaction energy profile illustrates:
- Reactants’ energy
- Transition state (activated complex)
- Products’ energy
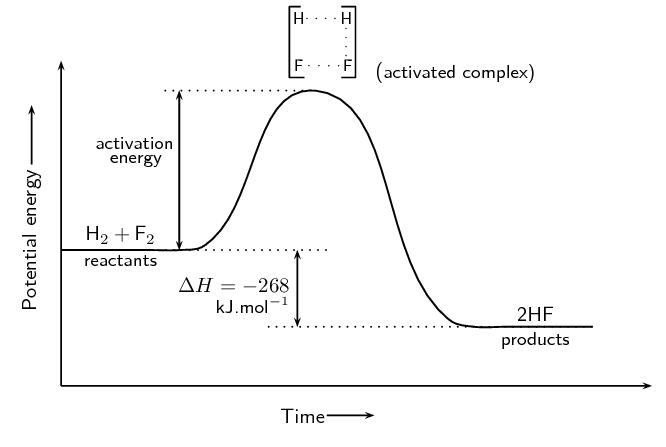
5.7 Introduction to Reaction Mechanisms
Reaction mechanisms describe the step-by-step process of a reaction. Identifying the rate-determining step (slowest step) helps find the overall reaction rate.
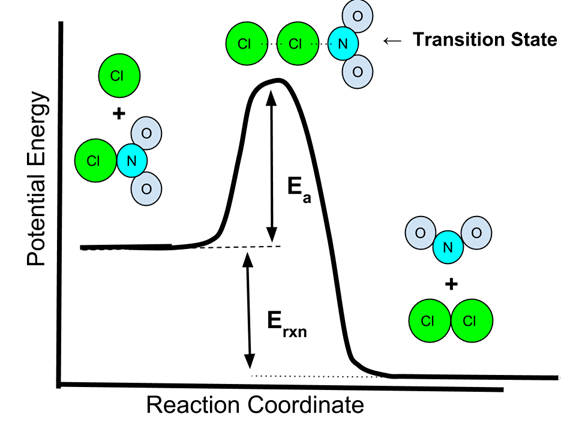
Image Courtesy of SoftSchools
5.8 Reaction Mechanism and Rate Law
Mechanisms reveal:
- Intermediates: Formed and consumed during the reaction.
- Catalysts: Lower activation energy without being consumed.
5.9 Steady-State Approximation
This mathematical method simplifies reaction analysis by assuming the concentration of intermediates remains constant.
5.10 Multistep Reaction Energy Profile
A multistep reaction profile shows energy changes for each step, illustrating activation energies, transition states, and overall energy changes.
5.11 Catalysis
Catalysts increase reaction rates by providing a lower-energy pathway. Enzymes are natural catalysts in biological systems.
Key Vocabulary
- Kinetics: Study of reaction rates and factors affecting them.
- Rate of Reaction: Change in reactant or product concentration per unit time.
- Rate Law: Relationship between reaction rate and reactant concentrations.
- Activation Energy (Ea): Minimum energy needed for a reaction.
- Catalyst: Substance increasing reaction rate without being consumed.
- Collision Model: Theory that reactions occur through molecular collisions.
- Intermediate: Species formed during the reaction but not present in the final products.
- Steady-State Approximation: Simplifies reactions by assuming constant intermediate concentration.
Practice Problem Example
Problem: The reaction A + B → C follows a rate law: Rate = k[A]²[B]. If the concentration of A is doubled while B is unchanged, by what factor does the rate increase?
Solution: Doubling [A] results in:
New rate=k[2A]2[B]=4k[A]2[B]
The rate increases by a factor of 4.
Conclusion
Understanding Kinetics provides insight into reaction rates, mechanisms, and energy changes. By mastering rate laws, collision theory, and catalysis, you can predict and influence chemical processes with precision. Keep practicing, and you’ll excel in this essential AP Chemistry unit!







