Mastering Chemical Reactions: Identifying and Understanding Acid-Base, Redox, and Precipitation Reactions
Introduction
Chemical reactions play a pivotal role in transforming matter, shaping the world around us through processes as simple as rust formation or as complex as combustion. In this comprehensive guide, we’ll delve deep into three key types of reactions: acid-base reactions, redox (oxidation-reduction) reactions, and precipitation reactions. By the end, you’ll gain insights into how these reactions work, when they occur, and how to identify and balance them accurately.
Types of Chemical Reactions Explained
1. Acid-Base Reactions
Acid-base reactions involve the transfer of a proton (H⁺) from one molecule (acid) to another (base). These reactions are common and often lead to the formation of a salt and water. For example:
HCl (acid) + NaOH (base) → NaCl (salt) + H₂O (water)
This type of reaction is essential in various industrial and biological processes. In AP Chemistry, you’ll explore how to use indicators to identify endpoints in titrations and how to calculate molar concentrations of acids or bases during these reactions.
2. Oxidation-Reduction (Redox) Reactions
In redox reactions, electrons are transferred between substances, changing their oxidation states. The substance that loses electrons is oxidized, while the substance that gains electrons is reduced. Combustion reactions, such as burning methane, are common examples of redox processes.
- Example: Combustion of methane (CH₄): CH₄ + 2O₂ → CO₂ + 2H₂O
You’ll learn to track electron transfers, balance redox reactions, and identify reducing and oxidizing agents. We’ll dive deeper into redox concepts later in this unit.
3. Precipitation Reactions
Precipitation reactions occur when two aqueous solutions combine to form an insoluble product or precipitate. This reaction type is often accompanied by a visual change, such as the formation of a solid. Understanding solubility rules is critical in predicting and identifying precipitates.
- Example: 2NaCl (aq) + Pb(NO₃)₂ (aq) → 2NaNO₃ (aq) + PbCl₂ (s)
Here, PbCl₂ is the precipitate, as it is insoluble in water.
Deep Dive into Precipitation Reactions
When ions in aqueous solutions react, they may produce an insoluble or slightly soluble solid known as a precipitate. Predicting whether a precipitate will form depends on knowing solubility rules. The AP Chemistry exam emphasizes that all sodium, potassium, ammonium, and nitrate salts are soluble, but it’s helpful to familiarize yourself with other common rules as well.
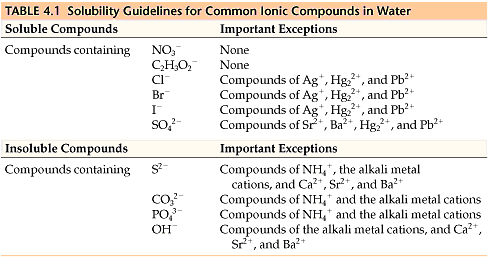
Writing Net Ionic Equations for Precipitation Reactions
Steps to Writing Net Ionic Equations
- Determine Solubility: Identify which compounds are soluble and which are insoluble.
- Balance the Chemical Equation: Ensure the number of atoms of each element is equal on both sides.
- Write the Complete Ionic Equation: Dissociate all soluble compounds into ions.
- Eliminate Spectator Ions: Spectator ions do not participate in the actual reaction. Remove them to form the net ionic equation.
Example Problem: 2NaCl (aq) + Pb(NO₃)₂ (aq) → 2NaNO₃ (aq) + PbCl₂ (s)
- Complete Ionic Equation: 2Na⁺ (aq) + 2Cl⁻ (aq) + Pb²⁺ (aq) + 2NO₃⁻ (aq) → 2Na⁺ (aq) + 2NO₃⁻ (aq) + PbCl₂ (s)
- Net Ionic Equation: Pb²⁺ (aq) + 2Cl⁻ (aq) → PbCl₂ (s)
The net ionic equation shows only the ions involved in forming the precipitate, excluding spectator ions.
Concentration of Ions After a Reaction
Understanding the concentration of ions after a reaction is crucial for predicting outcomes. Let’s walk through an example.
Example Problem: Concentration of Ions After Precipitation Reaction
Problem: 20.0 mL of 0.100 M NaCl (aq) reacts with 30.0 mL of 0.0400 M Pb(C₂H₃O₂)₂ (aq).
Part A: What is the mass of the solid formed?
Solution:
Write the Balanced Equation: 2NaCl (aq) + Pb(C₂H₃O₂)₂ (aq) → 2NaC₂H₃O₂ (aq) + PbCl₂ (s)
Calculate Moles:
- NaCl: 0.100 M × 0.020 L = 0.00200 moles
- Pb(C₂H₃O₂)₂: 0.0400 M × 0.030 L = 0.00120 moles
Identify the Limiting Reactant (LR): Perform stoichiometric calculations to find that NaCl is the LR.
Calculate Mass of Precipitate (PbCl₂): 0.00100 mol PbCl₂ × 278.2 g/mol = 0.278 g
Part B: Calculate Ion Concentrations After Reaction
- Chloride (Cl⁻): 0 M (used up)
- Sodium (Na⁺): 0.0400 M
- Acetate (C₂H₃O₂⁻): 0.0480 M
- Lead (Pb²⁺): 0.0040 M
Summary of Key Takeaways
- Acid-base reactions involve proton transfers.
- Redox reactions entail electron transfers.
- Precipitation reactions produce insoluble products.
- Net ionic equations simplify reactions by excluding spectator ions.
- Stoichiometry and limiting reactants are critical for calculating reaction products and remaining ion concentrations.
Conclusion
Understanding and identifying chemical reactions are central to mastering AP Chemistry. Whether you’re balancing equations, predicting products, or calculating ion concentrations, each concept builds your foundation for more advanced topics. Keep practicing, and you’ll become proficient at tackling even the toughest chemistry challenges!







