What Are Titrations and How Are They Used in Chemistry?
Written by SlyAcademy Team
Table of Contents
- What Are Titrations?
- Key Components in Titration
- Types of Titrations
- Understanding Acid-Base Titrations
- Carrying Out a Titration
- Graphical Representations of Titrations
- Simple Titration Calculations
- Titration Calculations Example
- Acids and Bases in Titrations
- Review Activity
- Conclusion
What Are Titrations?
Titrations are a common laboratory technique used to determine the unknown concentration of a chemical solution. This method involves two main substances: the titrant and the analyte.
Key Components in Titration
Titrant
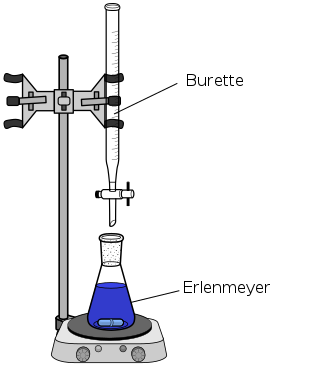
The titrant is a solution of known concentration. It is added carefully to the unknown solution using a burette, a long narrow tube with a stopcock that precisely controls the volume of titrant dispensed.
Analyte
The analyte is the solution with an unknown concentration. It is placed in an Erlenmeyer flask beneath the burette. You may sometimes hear it referred to as the titrand.
Types of Titrations
Acid-Base Titrations
In acid-base titrations, a titrant (either a strong acid or strong base) reacts with an unknown analyte concentration, often a weak acid or base. The endpoint is detected by a pH change, observed with an indicator or a pH meter.
Redox Titrations
These titrations determine the concentration of an oxidizing or reducing agent using color changes, electronic meters, or redox indicators.
Precipitation Titrations
In these titrations, a precipitate forms when two reactants combine, and the endpoint is indicated by a visual or instrumental change.
Complexation Titrations
These measure the concentration of a complexing agent, with endpoints indicated by color or absorption changes using spectrophotometric techniques.
Understanding Acid-Base Titrations
In this type of titration, the titrant (usually a strong acid or base) is added to the analyte (a weak acid or base) in an Erlenmeyer flask. The titration process is monitored using an indicator that changes color at the endpoint, signaling the equivalence point where moles of titrant equal moles of analyte.
Carrying Out a Titration
- Prepare the burette with the titrant. Record the initial volume.
- Measure the analyte and place it in the flask.
- Add indicator drops if necessary.
- Gradually add the titrant, stirring continuously, until the endpoint is observed (color change or pH shift).
Equivalence Point vs. Endpoint
- Equivalence Point: Moles of titrant equal moles of analyte; reaction is complete.
- Endpoint: The point where a visible change (e.g., color) occurs, indicating near equivalence.
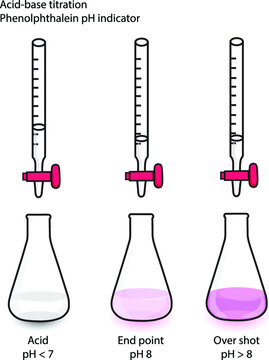
Image Courtesy of Adobe Stock
Graphical Representations of Titrations
Titration curves plot the relationship between the volume of titrant added and the pH of the analyte.
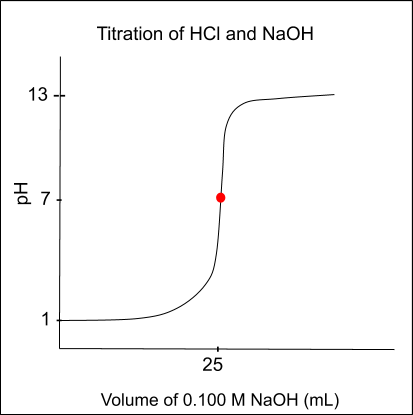
- Linear Region: Shows relatively stable pH as titrant is added.
- Inflection Point: Sharp change in slope, indicating the equivalence point.
- Endpoint: Typically observed as a visible change in the solution.
Simple Titration Calculations
Since the equivalence point is when moles of acid equal moles of base, we can use the equation:
MaVa = MbVb
Note: Adjust for mole ratios if not 1:1.
Titration Calculations Example
Problem: A solution of vinegar contains an unknown concentration of acetic acid, HC₂H₃O₂. A 25.0 mL sample of vinegar is titrated with 0.650 M NaOH. It takes 32.04 mL of titrant to reach the equivalence point. What is the concentration of HC₂H₃O₂?
Solution: HC₂H₃O₂ (aq) + NaOH (aq) → H₂O (l) + NaC₂H₃O₂ (aq)
Using MaVa = MbVb: (Ma)(25.0 mL) = (0.650 M)(32.04 mL) Ma=0.833M
Acids and Bases in Titrations
In the Brønsted-Lowry definition:
- Acids: Proton donors.
- Bases: Proton acceptors.
Example reaction: HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Review Activity
Identify the Acid, Base, and Their Conjugates
- H₂SO₄ (aq) + CH₃NH₂ (aq) → CH₃NH₃⁺ (aq) + HSO₄⁻ (aq)
- NH₃ (aq) + HNO₂ (aq) → NH₄⁺ (aq) + NO₂⁻ (aq)
Conclusion
Titrations are fundamental to determining solution concentrations. Mastering the titration process, from recognizing key components to performing calculations, provides a solid foundation for advanced chemical studies.







