Balancing Chemical Equations: The Essential Guide for Mastery
Written by SlyAcademy Team
Table of Contents
- Introduction to Balancing Chemical Equations
- Why Balancing Chemical Equations is Important
- Steps to Balance Chemical Equations
- Balancing Examples
- Practice Problems
- Conclusion
Introduction to Balancing Chemical Equations
Balancing chemical equations is a fundamental skill in chemistry. A balanced equation ensures that the law of conservation of mass is followed, indicating that the amount of matter remains constant in a closed system. This principle states that the total number of atoms of each element must remain the same before and after a reaction. Mastering this skill is crucial for anyone studying chemistry, particularly students preparing for AP Chemistry exams.
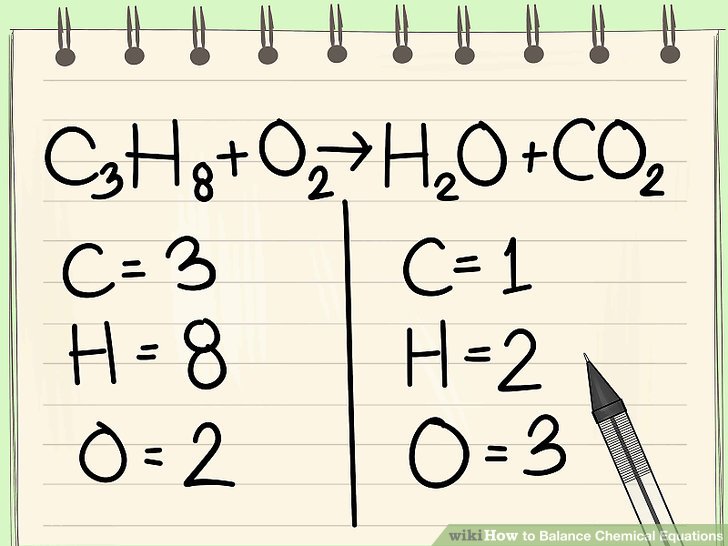
Image Courtesy of Quizizz
Why Balancing Chemical Equations is Important
Chemical reactions involve the transformation of reactants into products, often resulting in new substances with different properties. To accurately represent these transformations, chemists must ensure the number of atoms of each element is consistent on both sides of the equation. Here’s why balancing reactions is critical:
- Adherence to the Law of Conservation of Mass: In a closed system, matter cannot be created or destroyed.
- Accurate Representation of Reactions: Balanced equations allow chemists to understand the correct proportions of reactants and products.
- Predicting Reaction Outcomes: Properly balanced equations are necessary for calculating yields and understanding chemical behavior.
Steps to Balance Chemical Equations
Balancing chemical equations may seem challenging at first, but with practice, you can master this essential skill. Follow these general steps:
- Check if the Equation is Already Balanced: Start by counting the number of atoms of each element on both sides.
- Balance Elements Found in a Single Compound on Each Side: If an element appears only once on both sides, balance it first.
- Adjust Coefficients, Not Subscripts: Changing subscripts changes the chemical identity of the compound. Adjust coefficients (whole numbers in front of compounds) instead.
- Balance Elements Found in Multiple Compounds Last: Elements appearing in more than one compound on either side should be balanced afterward.
- Double-Check Your Work: Ensure the number of atoms of each element matches on both sides of the equation.
Balancing Examples
Example 1: Synthesis of Carbon Dioxide
Reaction:
CO (g) + O₂ (g) → CO₂ (g)
Check Initial Balance:
- Carbon (C): 1 on both sides (Balanced)
- Oxygen (O): 2 on the left (reactants) and 3 on the right (products)
Adjust Oxygen: Increase the coefficient of CO₂ to 2:
CO (g) + O₂ (g) → 2CO₂ (g)Adjust Carbon: Increase the coefficient of CO to 2 to balance carbon:
2CO (g) + O₂ (g) → 2CO₂ (g)Final Check:
- Carbon (C): 2 on both sides
- Oxygen (O): 4 on both sides
The equation is balanced!
Example 2: Synthesis of Lithium Nitride
Reaction:
Li (s) + N₂ (g) → Li₃N (s)
Check Initial Balance:
- Lithium (Li): 1 on the left, 3 on the right
- Nitrogen (N): 2 on the left, 1 on the right
Balance Lithium: Increase the coefficient of Li to 3:
3Li (s) + N₂ (g) → Li₃N (s)Balance Nitrogen: Increase the coefficient of Li₃N to 2:
3Li (s) + N₂ (g) → 2Li₃N (s)Re-Balance Lithium: Adjust the coefficient of Li to 6:
6Li (s) + N₂ (g) → 2Li₃N (s)Final Check:
- Lithium (Li): 6 on both sides
- Nitrogen (N): 2 on both sides
The equation is balanced!
Practice Problems
Try balancing these equations on your own:
- Na₃PO₄ + AgNO₃ → Ag₃PO₄ + NaNO₃
- Fe₂O₃ + CO → Fe + CO₂
- C₂H₆ + O₂ → CO₂ + H₂O (Combustion of Ethane)
- SO₂ + O₂ → SO₃ (Synthesis of Sulfur Trioxide)
- KClO₃ → KCl + O₂ (Decomposition of Potassium Chlorate)
Answers:
- Na₃PO₄ (aq) + 3AgNO₃ (aq) → Ag₃PO₄ (s) + 3NaNO₃ (aq)
- Fe₂O₃ (s) + 3CO (g) → 2Fe (s) + 3CO₂ (g)
- 2C₂H₆ (g) + 7O₂ (g) → 4CO₂ (g) + 6H₂O (l)
- 2SO₂ (g) + O₂ (g) → 2SO₃ (g)
- 2KClO₃ (s) → 2KCl (aq) + 3O₂ (g)
Conclusion
Balancing chemical equations is a key skill in understanding chemical reactions. By mastering this technique, you ensure accurate predictions, align with the law of conservation of mass, and deepen your comprehension of chemical processes. Practice consistently to build confidence and proficiency!







