What Is Light?
Scientists began understanding light by analyzing how substances emit or absorb light. The visible light we see is just one type of electromagnetic radiation, which is also known as radiant energy because it carries energy through space.
Light is carried in the form of photons, which are quantum particles that act as “force-carrying particles” for electromagnetic energy. For example, when you turn on a flashlight, photons shoot out, creating a beam of light. Photons are also involved in lasers and in many critical measurements like the Beer-Lambert law, which helps measure concentrations of solutions.
Fun Fact: The word LASER stands for Light Amplification by Stimulated Emission of Radiation!
Properties of Light
Light behaves both as a particle (photon) and a wave, a concept known as wave-particle duality. This is the same principle that applies to electrons, which also exist as both particles and waves.
When describing light as a wave, we refer to key properties:
- Amplitude: The vertical height of a wave from the midline, which determines the intensity or brightness of light. Higher amplitude means brighter light.
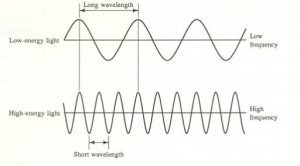
- Wavelength (λ): The distance between two peaks (or troughs) of a wave, measured in nanometers (nm) or meters. Wavelength determines the color of light.
- Frequency (ν): The number of wave cycles that pass a point in one second, measured in Hertz (Hz) or s⁻¹. Frequency is directly related to the speed of the wave; the higher the frequency, the faster the wave moves. Wavelength and frequency are inversely proportional, meaning a longer wavelength results in a lower frequency and vice versa. This relationship is expressed in the formula:
where c is the speed of light (approximately 3.00×108m/s).
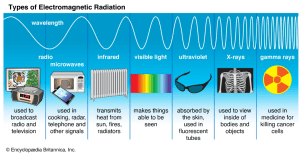
The Electromagnetic Spectrum 🌈
The electromagnetic spectrum includes all wavelengths of electromagnetic radiation, from very short gamma rays to very long radio waves.
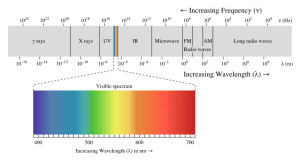
Key trend: Shorter wavelength = Higher frequency.
The spectrum can be broken down as follows:
- Gamma rays (γ): Shortest wavelength, highest frequency, dangerous because of ionizing radiation.
- X-rays: Longer wavelength than gamma rays, used in medical imaging.
- Ultraviolet (UV) radiation: Harmful at high doses, causes sunburn and increases cancer risk.
- Visible light: The light humans can see, with wavelengths ranging from 400 nm (purple) to 700 nm (red).
- Infrared radiation (IR): Experienced as heat, emitted by warm objects.
- Microwaves: Used in satellite communication and microwave ovens.
- Radio waves: Longest wavelength, used for radio, TV, and cell signals.
The Electromagnetic Spectrum Diagram
| Type of Radiation | Wavelength | Practical Use |
|---|---|---|
| Gamma rays | Shortest | Medical treatment, nuclear energy |
| X-rays | Short | Imaging bones and organs |
| Ultraviolet (UV) rays | Short | Causes sunburn, sterilization |
| Visible light | Medium | Enables sight, transitions at atomic energy levels |
| Infrared (IR) | Medium-long | Heat radiation, remote controls |
| Microwaves | Longer | Cooking, satellite communication |
| Radio waves | Longest | TV and radio transmission, mobile communication |
Spectroscopy: The study of how radiant energy interacts with matter, using the electromagnetic spectrum to examine energy transitions at the molecular and atomic levels.
This provides a comprehensive overview of light, wave-particle duality, and the electromagnetic spectrum for AP Chemistry.