Unit 3.3: Environmental Impacts on Enzyme Function
Changes in the Environment Affect Enzyme Activity
Enzymes are essential proteins that catalyze chemical reactions in living organisms. However, environmental factors, such as temperature, pH, and concentration, can significantly affect how well an enzyme functions. When proteins are denatured, they lose their function, and while some enzymes can undergo renaturation (regaining their function), this is not always the case. Think of it like curling your hair—it will eventually return to its original form (renaturation), but boiling an egg is a permanent change (nonreversible denaturation). Let’s explore how these factors impact enzyme function.
Temperature
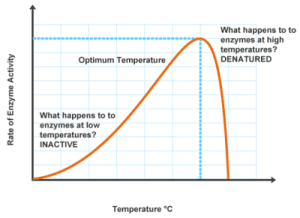
Temperature plays a critical role in enzyme activity, both in speeding up and slowing down reactions. At low temperatures, molecules move slowly, reducing the chances of enzymes and substrates colliding, thus slowing down the rate of reaction.
As temperatures increase, molecular movement accelerates, leading to more frequent collisions between enzymes and substrates. This generally results in an increased rate of reaction—up to a certain threshold. Beyond this optimal temperature, the enzyme can begin to denature. When the temperature is too high, the bonds that maintain the enzyme’s three-dimensional shape start to break, leading to loss of the active site and, consequently, its function. Remember, enzyme structure is crucial for its function; without the correct structure, the enzyme cannot catalyze reactions effectively.
Most enzymes in the human body function best around our body temperature, which is approximately 97-99 degrees Fahrenheit (or around 37°C). If the temperature falls too far above or below this range, enzyme performance declines, and life-sustaining reactions may not occur efficiently.
pH
pH measures the concentration of hydrogen ions in a solution. A low pH indicates a high concentration of hydrogen ions, meaning the solution is acidic, while a high pH means fewer hydrogen ions, indicating the solution is basic. Every enzyme has an optimal pH at which its activity is highest.
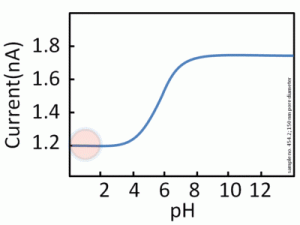
When the pH deviates from the enzyme’s optimal range, the enzyme’s activity slows down and may eventually lead to denaturation. The hydrogen bonds that are vital for maintaining an enzyme’s shape are disrupted by changes in pH, leading to changes in the enzyme’s structure and therefore its function.
Most enzymes have an optimal pH of around 7. However, some, such as pepsin (a digestive enzyme found in the stomach), function best in highly acidic environments (pH 2). Likewise, enzymes found in the lysosome are adapted to operate optimally at lower pH levels.
Concentration
The concentration of enzymes and substrates also impacts the overall rate of reaction. An increased concentration of either enzyme or substrate enhances the likelihood of collisions, thereby increasing the reaction rate. However, if the concentration of one exceeds the other, it can act as a limiting reagent, restricting the reaction rate.
If both enzyme and substrate concentrations are sufficiently high, the chance of them meeting and forming an enzyme-substrate complex rises, leading to an efficient reaction. However, if only one is in low supply, it limits how many reactions can occur at a time.
Inhibitors
Inhibitors are molecules that reduce enzyme activity, but they do not necessarily denature the protein. Instead, they impact how efficiently enzymes function.
Competitive Inhibitors: These molecules bind directly to the active site, preventing the intended substrate from binding. This decreases the likelihood of the enzyme catalyzing its intended reaction but does not alter the enzyme’s structure.
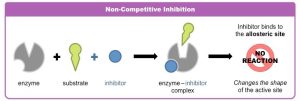
Noncompetitive Inhibitors: These molecules bind to a different part of the enzyme, which indirectly alters the active site, preventing the substrate from binding. This change in structure reduces enzyme activity.
Both competitive and noncompetitive inhibitors can be reversible or irreversible, depending on the strength and nature of their interactions with the enzyme.
Conclusion
Enzymes are sensitive proteins that require specific conditions to function optimally. Factors such as temperature, pH, concentration, and the presence of inhibitors can significantly alter enzyme activity—either enhancing their function or causing them to lose their catalytic abilities. The human body maintains strict control over temperature and pH to ensure that enzymes function at their best. When these conditions deviate too far from the optimal range, enzyme activity may decline, and essential biological processes may be disrupted.