Unit 3.1: Enzyme Structure
Understanding Enzymes: The Key to Life’s Reactions
Enzymes are essential to maintaining the highly complex organization of living systems. They act as biological catalysts, enabling and speeding up chemical reactions without being consumed in the process. These specialized proteins play an indispensable role in many cellular processes, such as metabolism, cell division, and gene expression, which are vital for survival and growth. But how do they work? Let’s dive into the details to understand enzyme structure, their function, and how they contribute to cellular efficiency.
How Enzyme Structure Relates to Function
The structure of an enzyme is critical to its function. Enzymes are composed of one or more polypeptide chains, which are long sequences of amino acids linked together. The sequence and arrangement of amino acids in these chains determine the enzyme’s unique structure, which is essential for its catalytic activity.
Enzymes possess four levels of structure:
Primary Structure: The linear sequence of amino acids in the polypeptide chain forms the enzyme’s primary structure. It represents the order in which amino acids are connected, much like a unique arrangement of letters in a sentence.
Secondary Structure: The polypeptide chain folds into regular patterns, forming alpha-helices or beta-sheets through hydrogen bonding. This secondary structure provides stability to the enzyme and contributes to its overall three-dimensional shape.
Tertiary Structure: The secondary structure folds further into a more complex three-dimensional shape, forming the enzyme’s tertiary structure. This folding creates the active site where the substrate interacts and the reaction takes place.
Quaternary Structure: Some enzymes consist of multiple polypeptide chains that come together to form a functional enzyme complex. The quaternary structure describes how these subunits interact and assemble to create a functional enzyme.

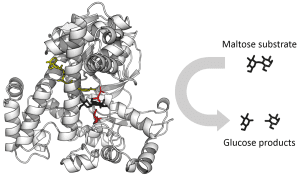
The enzyme’s three-dimensional structure is crucial because it determines the shape of the active site—the specific area where substrates bind and chemical reactions occur. This shape allows enzymes to interact specifically with their substrates, making them highly selective in the reactions they catalyze.
Active Site: The Heart of Enzyme Activity
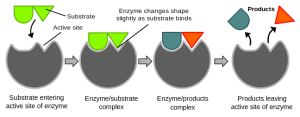
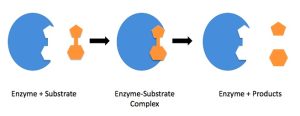
The active site is the region on the enzyme where substrate molecules bind and undergo a chemical reaction. This site is typically a cleft or groove on the enzyme’s surface and is often lined with specific amino acids that interact directly with the substrate.
The active site provides a unique environment, perfectly shaped to hold the substrate in a specific orientation. This binding occurs because the active site’s shape and chemical properties are complementary to those of the substrate. This phenomenon is often called “lock-and-key” specificity, where the enzyme (lock) is tailored to fit its particular substrate (key).
However, the enzyme’s specificity does not make it rigid. In fact, enzymes exhibit some flexibility that enhances their function, which brings us to the induced fit model.
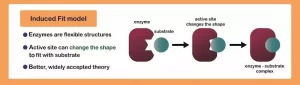
Induced Fit: Enhancing Catalytic Efficiency
The induced fit mechanism describes how enzymes change their shape slightly upon binding to the substrate. Rather than being a perfect fit from the start, the enzyme undergoes subtle structural adjustments to achieve an even tighter fit after initial contact with the substrate—similar to how a glove molds itself to a hand once you put it on.
This conformational change allows for a better fit between the enzyme and the substrate, which increases the efficiency of the reaction. As the substrate enters the active site, the enzyme’s amino acid residues adjust to create an optimal environment for the reaction to proceed. The tighter fit allows for more efficient formation of the transition state, which is the high-energy intermediate that leads to the product.
Induced fit also contributes to substrate specificity. Although enzymes can adjust to fit a variety of substrates, they only do so effectively when the substrate fits well enough to cause the correct conformational change. Therefore, only the best-fitting substrates are catalyzed with high efficiency.
Enzyme Regulation: Controlling Reaction Rates
Enzymes are not always working at full speed; they need to be regulated. Cells use several mechanisms to control enzyme activity and ensure that chemical reactions occur at the right time and rate.
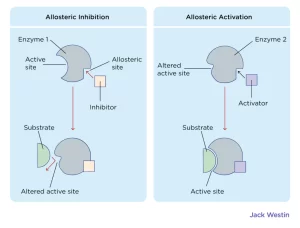
Allosteric Regulation: Allosteric regulation occurs when a molecule binds to a specific site on the enzyme other than the active site (known as the allosteric site). This binding changes the enzyme’s shape, either activating or inhibiting its activity. Allosteric regulation is essential for maintaining homeostasis in cells by ensuring that enzymes are active only when needed.
Competitive Inhibition: Competitive inhibitors are molecules that resemble the substrate and compete for binding to the enzyme’s active site. When a competitive inhibitor binds, it prevents the substrate from entering, thereby slowing down the reaction rate.
Noncompetitive Inhibition: Noncompetitive inhibitors bind to an enzyme at a location other than the active site. This binding changes the enzyme’s shape, rendering the active site less effective or inactive. Unlike competitive inhibitors, noncompetitive inhibitors do not directly block substrate binding but instead alter enzyme function by changing its structure.
Importance of Enzymes in Cellular Processes
Enzymes are indispensable for the proper functioning of biological systems. They lower the activation energy required for chemical reactions, making them proceed at a rate necessary for life. Without enzymes, essential cellular reactions would occur too slowly to sustain life.
For example, enzymes are involved in:
Metabolism: Enzymes facilitate the breakdown of nutrients, converting them into usable energy (ATP) through metabolic pathways like glycolysis and the citric acid cycle.
DNA Replication: Enzymes such as DNA polymerase catalyze the replication of DNA, allowing cells to divide and pass on genetic information.
Signal Transduction: Enzymes play a role in cell signaling by phosphorylating proteins, which alters their activity and transmits signals within the cell.
The Bigger Picture: How Enzymes Maintain Life’s Order
Living organisms are highly ordered systems, and enzymes play a key role in maintaining this order. By speeding up chemical reactions, enzymes ensure that cells can efficiently convert energy, grow, reproduce, and respond to environmental changes. The continuous input of energy—made possible by enzymatic reactions—allows living systems to fight against the natural tendency toward disorder (entropy) as dictated by the laws of thermodynamics.
Enzymes are not just passive players but dynamic molecules that respond to changes in their environment. Whether it’s the substrate concentration, temperature, pH, or the presence of inhibitors, enzymes are at the core of how cells adapt and regulate their internal processes to stay alive.
Conclusion
Understanding the structure and function of enzymes is crucial to understanding life at a molecular level. Enzymes are not only catalysts but also regulators, maintaining the delicate balance required for cellular survival and function. Their ability to bind to substrates, change shape for induced fit, and be regulated through various mechanisms makes them powerful tools for life.
As we continue to explore enzyme structure and function, we unlock deeper insights into the biochemistry of life—insights that have far-reaching implications in medicine, agriculture, and biotechnology.