AP Biology 1.4: Properties of Biological Macromolecules
Welcome to AP Biology Unit 1.4! In this section, we dive into the properties of biological macromolecules—carbohydrates, lipids, proteins, and nucleic acids. Understanding these properties is key to grasping how these molecules support life processes. 🌱
Macromolecules Overview
Macromolecules are large molecules composed of two or more polymers combined together (macro = large). They serve as the building blocks for cells and perform a wide range of vital functions in living organisms.
Carbohydrates
Carbohydrates are sugars or polymers of sugars and usually end in the suffix -ose. They contain multiple hydroxyl groups and a carbonyl group. The position of the carbonyl group determines if a sugar is an aldose (carbonyl at the end) or a ketose (carbonyl in the middle).
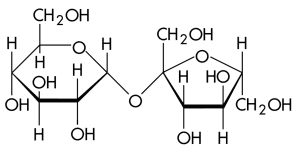
Monosaccharides are simple sugars with a general formula of (CH₂O)_n and typically exist in a 1:2:1 ratio. Glucose (C₆H₁₂O₆) is the most common monosaccharide and plays a significant role in glycolysis. Another common monosaccharide is fructose.
Carbohydrates are crucial for storing energy and providing structure. As with all macromolecules, their structure determines their function.
Lipids
Lipids are unique among macromolecules as they do not form polymers. Lipids are nonpolar molecules that are hydrophobic and do not have an affinity for water. Lipids are made up of hydrocarbons and are an important storage molecule, providing 9 calories per gram. They are essential for insulation in animals.
Fats are made of glycerol and three fatty acids joined by an ester bond, forming a triglyceride.
Saturated and Unsaturated Fatty Acids
Saturated Fatty Acids: No double bonds between carbon atoms, saturated with hydrogen atoms, usually solid at room temperature (e.g., lard and butter).
Unsaturated Fatty Acids: One or more double or triple bonds between carbon atoms, usually liquid at room temperature (e.g., vegetable oils).
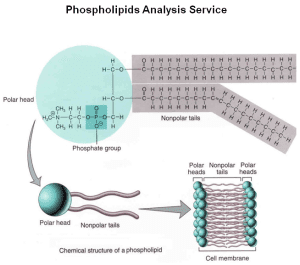
Phospholipids
Phospholipids consist of one glycerol, two fatty acids, and one phosphate group. They are a major component of the cell membrane, forming the phospholipid bilayer. The head (glycerol and phosphate) is hydrophilic, while the tail (fatty acids) is hydrophobic. When added to water, phospholipids assemble into a bilayer structure, which is key for the cell membrane.
Steroids
Steroids have a carbon skeleton made up of four fused rings. They are classified as lipids due to their hydrophobic nature. Cholesterol is an example of a steroid that plays a crucial role in animal cell membranes.
Proteins
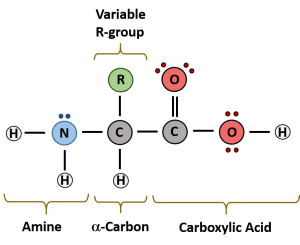
Proteins are made from 20 different amino acids joined by peptide bonds (also called polypeptides). The chemical and physical properties of the amino acids determine the protein’s shape and, consequently, its function.
When two amino acids join through dehydration synthesis, it is called a dipeptide. Polymers of amino acids are called polypeptides, and they fold into complex shapes to form functional proteins.
Each amino acid consists of an amino group, a carboxyl group, and an R group. The R group determines the properties of each amino acid (whether it is hydrophobic, hydrophilic, or ionic). The structure and properties of the R group affect how the protein folds and functions.
Nucleic Acids
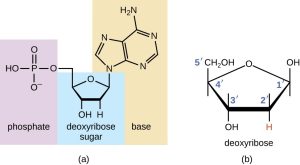
Nucleic acids are made up of carbon, hydrogen, oxygen, nitrogen, and phosphorus. The monomers of nucleic acids are nucleotides, which consist of a five-carbon sugar, a phosphate group, and a nitrogenous base (adenine, thymine, guanine, cytosine, uracil).
Nucleic acids store and transmit genetic information and are found in both DNA and RNA. While DNA stores genetic information, RNA helps in protein synthesis.
Big Fat Summary
Macromolecules are the core of life. Here is a summary to help you remember the properties:
| Macromolecule | Monomer | Polymer | Linkage Bond | Elements |
|---|---|---|---|---|
| Carbohydrates | Monosaccharide | Polysaccharide | Glycosidic bond | C, H, O |
| Proteins | Amino acid | Polypeptide | Peptide bond | C, H, O, N, S |
| Nucleic Acids | Nucleotide | DNA, RNA | Sugar-phosphate bond | C, H, O, N, P |
| Lipids | No true polymer | Phospholipids | Ester bonds | C, H, O, P |
Study Resources
For a more in-depth exploration, check out the AP Bio Unit 1 replays or watch the 2021 Unit 1 Cram session. Understanding the properties of biological macromolecules is fundamental for understanding how they contribute to cellular function and structure.
Mastering these macromolecules will give you a solid foundation in biology, as they are the key players in the molecular processes of life!