AP Biology 1.3: Introduction to Biological Macromolecules
Welcome to AP Biology Unit 1.3! In this section, we explore biological macromolecules and the chemical bonds that hold them together. Understanding these macromolecules and their structure helps us understand how life is built and functions at the molecular level.
Chemical Bonds
Covalent Bonds
Covalent bonds involve the sharing of electrons between atoms. A molecule formed by two or more atoms bonded covalently is known as a covalent compound. A single bond (sharing one pair of electrons) is represented by one line between atoms, while a double bond (sharing two pairs of electrons) is represented by two lines.
The electronegativity of an atom is its attraction for the shared electrons in a covalent bond. Examples of covalent compounds include methane (CH₄), carbon monoxide (CO), and iodine monobromide (IBr).
Ionic Bonds
Ionic bonds involve the transfer of electrons between atoms, creating electrostatic attraction between the resulting positively and negatively charged ions. These ionic compounds, often referred to as salts, are held together by the electrostatic force between the ions. Examples include sodium chloride (NaCl) and lithium fluoride (LiF).
Metallic Bonds
Metallic bonds occur in metals, where there is an attraction between metal ions and delocalized, or “free,” electrons. Examples of elements that form metallic bonds include iron, cobalt, silver, gold, platinum, copper, and zinc.
Polymers and Monomers
A polymer is a long molecule composed of many smaller molecules called monomers, which are bonded together covalently. Lipids are one of the major biological macromolecules that do not typically form polymers.
Monomers are small building block molecules that combine to form polymers. For example, amino acids are monomers that join together to create proteins, a type of polymer.
Covalent Bonds in Macromolecules
Nonpolar Covalent Bonds: These involve equal sharing of electrons, leading to an even distribution of charge. Nonpolar covalent bonds occur between atoms with similar electronegativities.
Polar Covalent Bonds: In these bonds, electrons are shared unequally, causing a partial positive or partial negative charge on each atom or molecule. Polar bonds are found between atoms with different electronegativities.
Intra- and Intermolecular Bonds
Intramolecular Bonds: These bonds exist within a molecule and include covalent bonds.

Intermolecular Bonds: These bonds form between molecules. One of the most important intermolecular bonds in biology is the hydrogen bond. Hydrogen bonds form when a hydrogen atom bonded to an electronegative atom is attracted to another electronegative atom, such as fluorine, nitrogen, or oxygen. It’s important to note that hydrogen bonds are not covalent bonds; they are weaker attractions that play a significant role in biological processes.
Dehydration Synthesis & Hydrolysis
Dehydration Synthesis
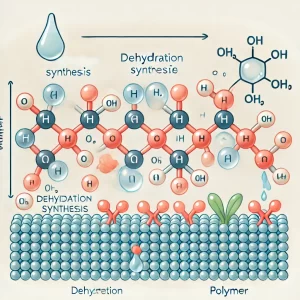
Dehydration synthesis is the process through which monomers combine to form polymers by removing a molecule of water. During this condensation reaction, one monomer donates OH⁻ and another donates H⁺, forming H₂O. This process requires energy (making it endergonic) and enzymes, and it builds complexity (anabolic—small molecules binding to form larger molecules).
Hydrolysis
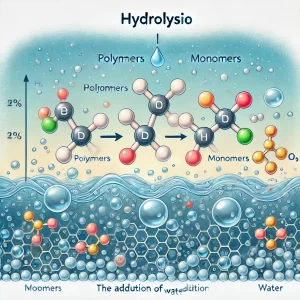
Hydrolysis is the process of breaking down polymers into monomers by adding water. It uses H₂O to split bonds, resulting in the formation of H⁺ and OH⁻ groups. This process releases energy (exergonic) and also requires enzymes, reducing complexity (catabolic).
Study Resources
For more detailed exploration, check out the AP Bio Unit 1 replays or watch the 2021 Unit 1 Cram session. Understanding biological macromolecules and their chemical bonds is fundamental to understanding the structure and function of cells and life.
By learning about chemical bonds, polymers, and monomers, you’re building a strong foundation for exploring the molecular processes that are crucial for living organisms!