
Acids and Bases

ACT

AP

AP Art and Design
AP Chemistry

AP Computer Science A

AP Computer Science Principles

AP English Language

AP English Literature

AP European History

AP Physics 1

AP Physics 2

AP Physics C : E & M
AP Physics C: Mechanics

AP Psychology (2025)

AP Score Calculators

AP World History: Modern

Applications of Thermodynamics

AQA

Artificial intelligence (AI)
Atomic Structure and Properties

Banking and Finance
Basic Electronics
Biology

Business Ideas
Calculator

Capacitors

ChatGPT

Chemical Reactions

Chemistry

Circular Motion and Gravitation

Colleges Rankings

Computer Science

Conversion Tools

Cosmetic Procedures

Cryptocurrency
DC Circuits

Digital SAT

Disease

Dynamics (Physics)

Edexcel
Electric Charge and Electric Force

Energy

English

Environmental Science
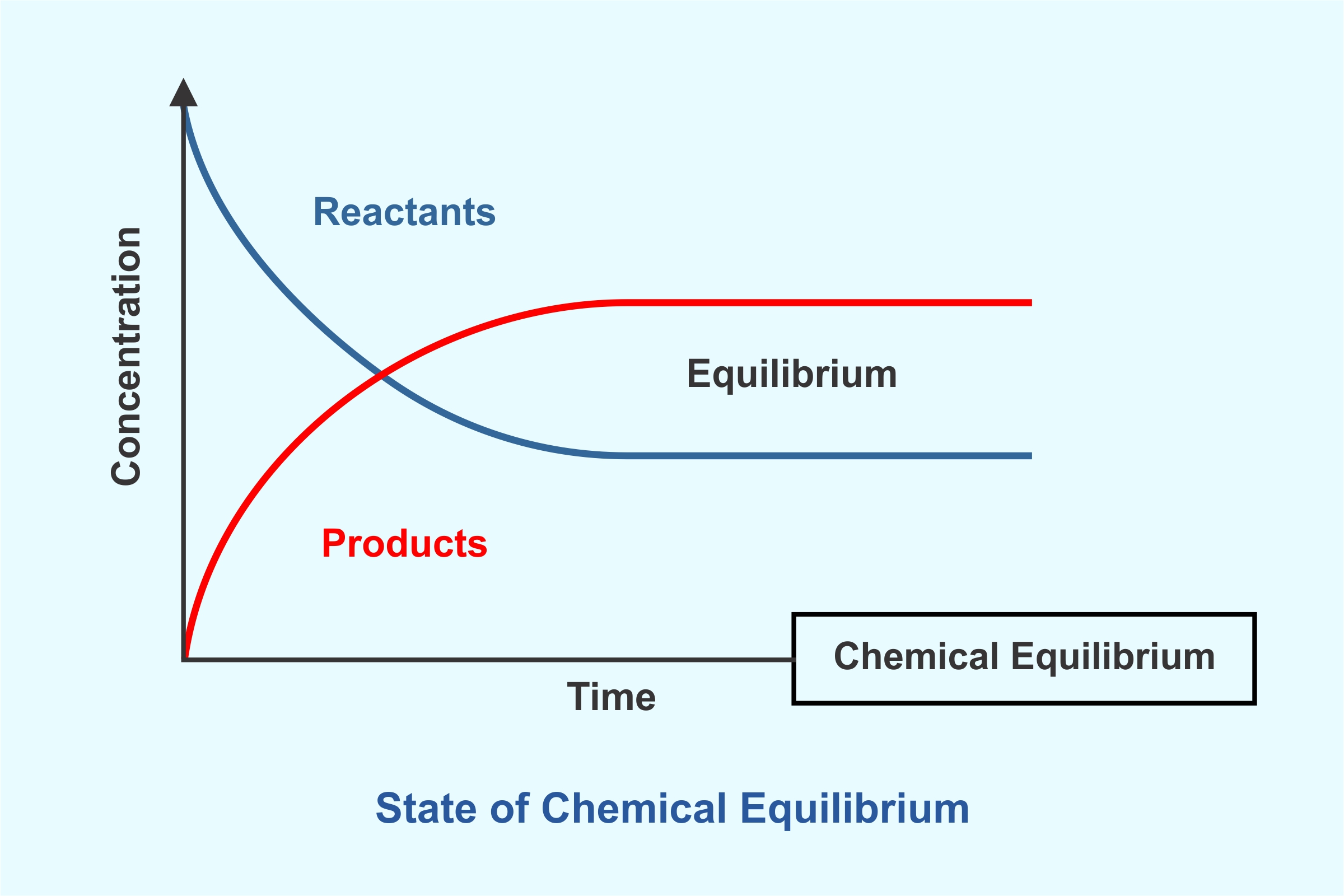
Equilibrium

Etiology

Exam Skills

Exam Updates

Finance

Fitness & Wellness
Free Learning Resources

GCSE

General Guides

Health

History and Social Sciences

IB

IB BUSINESS MANAGEMENT SL
IB MATH AAHL

IGCSE

Image Converters

IMF

Intermolecular Forces and Properties
Kinematics

Kinematics (Physics)

Math

Mechanical Waves and Sound

Mental Health

Molecular and Ionic Compound Structure and Properties

Momentum

News

OCR

Past Papers
Periodic Table
Physics

Research Study

SAT

Schools
Sciences

Short Notes

Simple Harmonic Motion

Study Guides

Syllabus

Thermochemistry

Tools
Torque and Rotational Motion

Tutoring
Unit 1: Biological Bases of Behavior

Unit 1: Claims, Reasoning, and Evidence

Unit 1: Creative Development

Unit 1: Electrostatics

Unit 1: Fluids

Unit 1: Introduction to Short Fiction
Unit 1: Kinematics

Unit 1: Primitive Types

Unit 1: Renaissance and Exploration

Unit 1: The Global Tapestry

Unit 10: Recursion

Unit 2: Cognition
Unit 2: Conductors, Capacitors, Dielectrics

Unit 2: Data

Unit 2: Introduction to Poetry

Unit 2: Networks of Exchange

Unit 2: Newton’s Laws of Motion

Unit 2: Organizing Information for a Specific Audience

Unit 2: Reformation

Unit 2: Thermodynamics
Unit 2: Using Objects

Unit 3: Absolutism and Constitutionalism

Unit 3: Algorithms and Programming

Unit 3: Boolean Expressions and if Statements

Unit 3: Development and Learning
Unit 3: Electric Circuits

Unit 3: Electric Force, Field, and Potential

Unit 3: Introduction to Longer Fiction and Drama

Unit 3: Land-Based Empires

Unit 3: Perspectives and How Arguments Relate

Unit 3: Work, Energy, and Power

Unit 4: Character, Conflict, and Storytelling

Unit 4: Computer Systems and Networks
Unit 4: Electric Circuits

Unit 4: How writers develop arguments, intros, and conclusions

Unit 4: Iteration
Unit 4: Magnetic Fields

Unit 4: Scientific, Philosophical, and Political Developments

Unit 4: Social Psychology and Personality

Unit 4: Systems of Particles and Linear Momentum

Unit 4: Transoceanic Interconnections

Unit 5: Computing's Impact on Society

Unit 5: Conflict, Crisis, and Reaction in the Late 18th Century
Unit 5: Electromagnetism

Unit 5: How a writer brings all parts of an argument together
Unit 5: Magnetism and Electromagnetic Induction

Unit 5: Mental and Physical Health

Unit 5: Revolutions

Unit 5: Rotation

Unit 5: Structure and Figurative Language

Unit 5: Writing Classes

Unit 6: Array

Unit 6: Consequences of Industrialization

Unit 6: Geometric and Physical Optics

Unit 6: Industrialization and Its Effects

Unit 6: Literary Techniques in Longer Works

Unit 6: Oscillations

Unit 6: Position, Perspective, and Bias

Unit 7: 19th-Century Perspectives and Political Developments

Unit 7: ArrayList

Unit 7: Global Conflict

Unit 7: Gravitation

Unit 7: Quantum, Atomic, and Nuclear Physics

Unit 7: Societal and Historical Context

Unit 7: Successful and Unsuccessful Arguments

Unit 8: 20th-Century Global Conflicts

Unit 8: 2D Array

Unit 8: Advanced Techniques in Poetry

Unit 8: Cold War & Decolonization

Unit 8: Stylistic Choices
Unit 9: Cold War and Contemporary Europe

Unit 9: Developing a Complex Argument

Unit 9: Globalization

Unit 9: Inheritance

Unit 9: Nuanced Analysis

What is?
Applications of Thermodynamics
Unit 9: Applications of Thermodynamics in AP Chemistry focuses on the principles of energy, entropy, and Gibbs free energy to predict and analyze the spontaneity of chemical processes.

N
NUM8ERS
0
0
9.10 Electrolysis and Faraday's Law
November 18, 2024
Save

N
NUM8ERS
0
0
9.9 Cell Potential Under Nonstandard Conditions
November 18, 2024
Save

N
NUM8ERS
0
0
9.8 Cell Potential and Free Energy
November 18, 2024
Save
N
NUM8ERS
0
0
9.7 Galvanic (Voltaic) and Electrolytic Cells
November 18, 2024
Save

N
NUM8ERS
0
0
9.5 Free Energy and Equilibrium
November 18, 2024
Save

N
NUM8ERS
0
0
9.4 Thermodynamic and Kinetic Control
November 18, 2024
Save

N
NUM8ERS
0
0
9.3 Gibbs Free Energy and Thermodynamic Favorability
November 18, 2024
Save

N
NUM8ERS
0
0
9.2 Absolute Entropy and Entropy Change
November 18, 2024
Save

N
NUM8ERS
0
0
Unit 9 Review: Applications of Thermodynamics
November 18, 2024
Save


