9.6 Coupled Reactions: Making the Impossible Possible
What Are Thermodynamically Unfavorable Reactions?
So far in Unit 9, we’ve focused on thermodynamically favorable reactions—those for which ΔG° < 0 and K > 1. These reactions occur spontaneously, requiring no external input of energy. But what about processes that are not so cooperative? Thermodynamically unfavorable reactions have ΔG° > 0, making them nonspontaneous. Such reactions do not proceed without an input of energy.
Adding Energy to Drive Reactions
There are several ways to push nonspontaneous reactions to occur:
- External Energy Sources: Energy, such as electricity, can be used to power processes. For instance, electrolytic cells—which we will discuss in Unit 9.7—use electrical energy to drive redox reactions that would otherwise be nonspontaneous. An example is the process of charging a battery, where electrical input is used to “push” electrons and drive reactions in reverse.
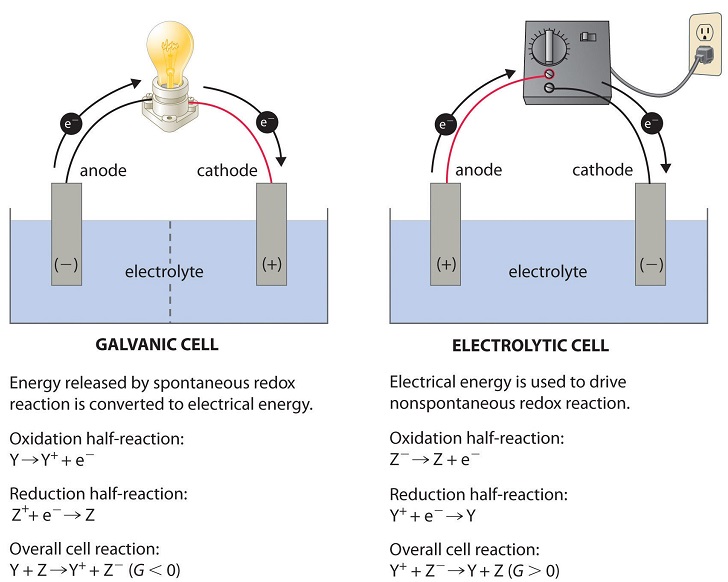
Image From LibreTexts/Electrochemistry/Electrolytic_Cells)
Coupled Reactions: The Secret to Spontaneity
Another way to make nonspontaneous reactions proceed is through coupled reactions. Coupling involves combining a nonspontaneous reaction with a spontaneous one that shares a common intermediate. This pairing can create a new, overall spontaneous reaction.
How It Works
Intermediates are species that are products of one step in a reaction mechanism and reactants in another. By using reactions with shared intermediates, we can transform nonspontaneous reactions into spontaneous processes. Let’s look at an example:
Example: Coupling Reactions
Consider this nonspontaneous reaction:
This reaction requires energy to proceed. However, we can find a spontaneous reaction that involves sulfur, like:
By coupling these reactions, we achieve:
- Nonspontaneous Reaction:
- Spontaneous Reaction:
- Combined Reaction:
The combined reaction is spontaneous (ΔG° < 0), demonstrating the power of coupling.
Applications in Biology and Chemistry
Coupled reactions are widely used in biological systems, including ATP hydrolysis. The conversion of ATP to ADP releases energy that can be coupled with nonspontaneous biological processes, driving essential reactions in living organisms.
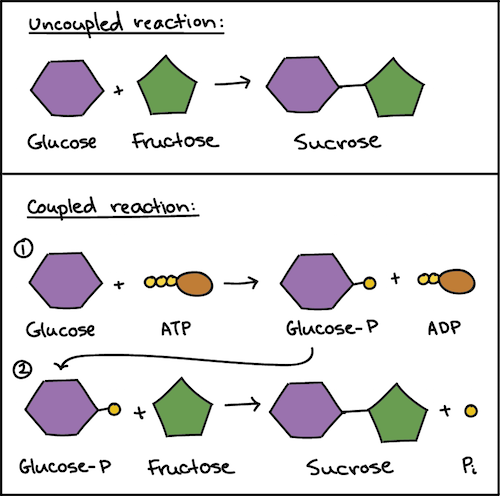
Image From Khan Academy
Practice Problem: Reaction Coupling
Given the following data, calculate ΔG° for the reaction:
- Reaction 1:
- Reaction 2:
Solution
- Multiply Reaction 2 by 3 to balance O2:
- Combine with Reaction 1:
The overall reaction is spontaneous with ΔG° = -108.3 kJ.
Key Takeaways
- Thermodynamically unfavorable reactions can proceed via coupling with spontaneous reactions.
- Coupled reactions play a vital role in biological and chemical systems, driving nonspontaneous processes forward.
- Understanding and using ΔG° calculations helps predict and manipulate reaction behavior.







