Understanding the Properties of Buffers: Why Buffers Are Crucial in Chemistry
In chemistry, buffers play an essential role in maintaining pH stability in solutions, making them critical in laboratory, industrial, and biological processes. This guide explores how buffers work, how they are formed, and why their unique properties make them indispensable.
Get it? Buffering? We’re hilarious. Image from GIPHY
What Are Buffers?
Buffers are solutions that resist changes in pH when small amounts of acids or bases are added. This ability to maintain pH stability is vital in many chemical and biological processes, such as enzyme activity, metabolic reactions, and industrial chemical reactions.
How Buffers Are Formed:
Buffers are created by combining a weak acid and its conjugate base or a weak base and its conjugate acid. This pairing allows the buffer solution to neutralize small amounts of added acids or bases, minimizing pH changes.
Key Requirements for Buffers
- Weak Acid or Base Requirement: A buffer must include a weak acid (HA) and its conjugate base (A-) or a weak base and its conjugate acid.
- Comparable Concentrations: For a buffer to be effective, the concentrations of the weak acid and its conjugate base (or vice versa) should be comparable.
Important Note: Strong acids and bases cannot form buffers. For example, a combination of HCl and NaCl does not produce a buffer because HCl is a strong acid, making Cl- an insignificant base.
Identifying Buffer Pairs: Examples
Let’s test your understanding with some examples. For each pair, we determine if they form a buffer:
- NaOH and Na+:
- Result: No. NaOH is a strong base, so Na+ is not a significant acid. No buffer is formed.
- CH₃COOH and Ca(CH₃COO)₂:
- Result: Yes. This pair forms a buffer. CH₃COOH (acetic acid) is a weak acid, and its conjugate base, CH₃COO-, is present from the dissociation of Ca(CH₃COO)₂.
- NH₃ and NH₄NO₃:
- Result: Yes. NH₃ (ammonia) is a weak base, and NH₄+ (from NH₄NO₃) is its conjugate acid, making this a buffer system.
- HI and I-:
- Result: No. HI is a strong acid, so I- is not a significant base and cannot form a buffer.
- Result: No. HI is a strong acid, so I- is not a significant base and cannot form a buffer.
What Makes Buffers Special?
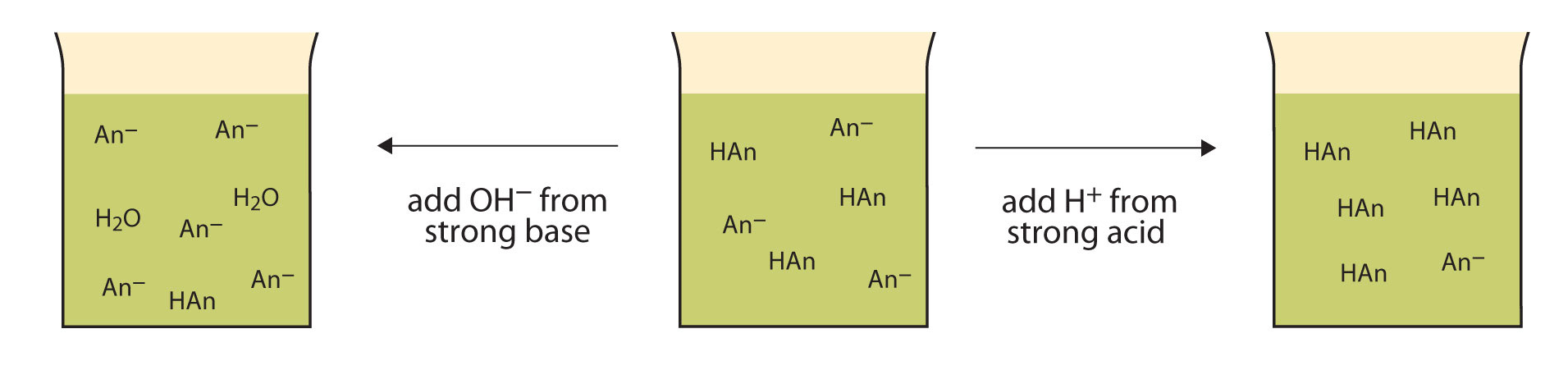
Buffers work by reacting with any added acid (H+) or base (OH-) to minimize changes in pH. Here’s how this works:
- Adding Acid (H+):
The conjugate base (A-) in the buffer reacts with the added H+ to form the weak acid (HA), reducing the impact on pH. - Adding Base (OH-):
The weak acid (HA) in the buffer reacts with OH- to form water (H₂O) and its conjugate base (A-), again reducing the impact on pH.
This property allows buffers to maintain pH stability in systems where precise pH control is essential, such as biological cells and industrial processes.
Buffer Capacity: How Much Can a Buffer Resist?
While buffers are excellent at resisting changes in pH, they have limitations known as buffer capacity. Once a buffer’s capacity is exceeded—meaning too much acid or base has been added—the solution will no longer effectively resist pH changes. Buffer capacity depends on:
- Concentration of Buffer Components: Higher concentrations of the weak acid and conjugate base (or vice versa) increase the buffer capacity.
- Ratio of Acid to Base: Buffers work best when the concentration of the weak acid equals that of the conjugate base (pH = pKa).
Image From LibreTexts
Practical Example: Understanding Buffer Action
Consider adding a strong acid, such as HCl, to a buffer containing CH₃COOH (weak acid) and CH₃COO- (conjugate base):
- The added H+ from HCl reacts with CH₃COO- to form CH₃COOH.
- Instead of significantly increasing the [H+] and lowering the pH, the reaction between H+ and CH₃COO- minimizes the pH change.
Similarly, when a strong base (OH-) is added, it reacts with CH₃COOH to form CH₃COO- and H₂O, again maintaining pH stability.
Why Buffers Matter
Buffers are crucial for maintaining pH balance in biological systems, chemical reactions, and industrial applications. For example, your blood contains a bicarbonate buffer system to maintain a stable pH essential for cell function and metabolism.
Quick Recap
- Buffers are solutions of a weak acid and its conjugate base (or vice versa) that resist pH changes.
- Buffer capacity is limited and depends on the concentration and ratio of buffer components.
- Buffers are widely used in biology, chemistry, medicine, and industry to maintain pH stability.