Energy in Chemical Reactions: Understanding Endothermic and Exothermic Processes
In our previous discussion on chemical kinetics, we delved into the collision model, which emphasizes that for a reaction to occur, collisions between molecules must have sufficient energy and correct orientation. Now, let’s focus on the energy aspect of reactions and explore what happens on a potential energy diagram during a chemical reaction.
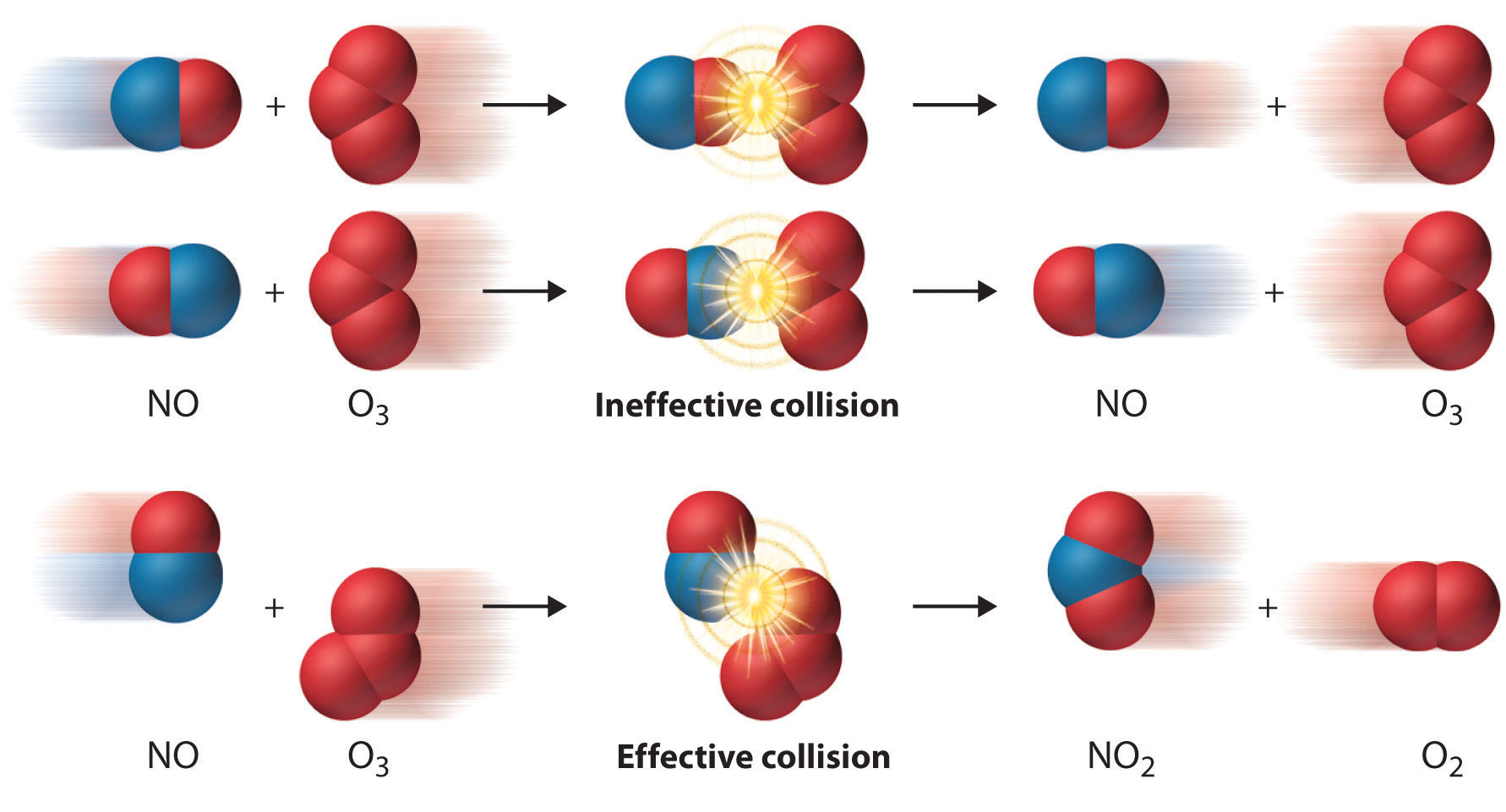
Image Courtesy
What is an Elementary Reaction?
Before diving deeper, let’s clarify elementary reactions. An elementary reaction is the simplest type of chemical reaction, occurring in a single step and involving a specific set of molecules or atoms. It is fundamental to understanding complex reaction mechanisms and plays a key role in kinetics.
Examples of Elementary Reactions:
- The combination of hydrogen and oxygen to form water.
- The decomposition of ozone into oxygen molecules.
- The ionization of a gas.
Endothermic vs. Exothermic Reactions: Understanding Potential Energy Graphs
A potential energy (PE) diagram shows how the energy of a system changes during a reaction. The energy can either be absorbed (endothermic reaction) or released (exothermic reaction). Here’s how to identify and differentiate them:
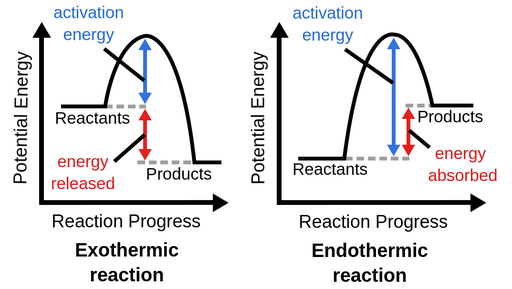
Image Courtesy of Labster Theory
1. Endothermic Reactions
- Definition: In an endothermic reaction, the potential energy of the products is higher than that of the reactants.
- Energy Change: Energy is absorbed from the surroundings.
- Equation Representation: Reactants+Energy→Products
- Example: The melting of ice into water.
Image Courtesy of Labster Theory
2. Exothermic Reactions
- Definition: In an exothermic reaction, the potential energy of the reactants is higher than that of the products.
- Energy Change: Energy is released into the surroundings, often as heat or light.
- Equation Representation: Reactants→Products+Energy
- Example: Combustion of methane.
Image Courtesy of Labster Theory
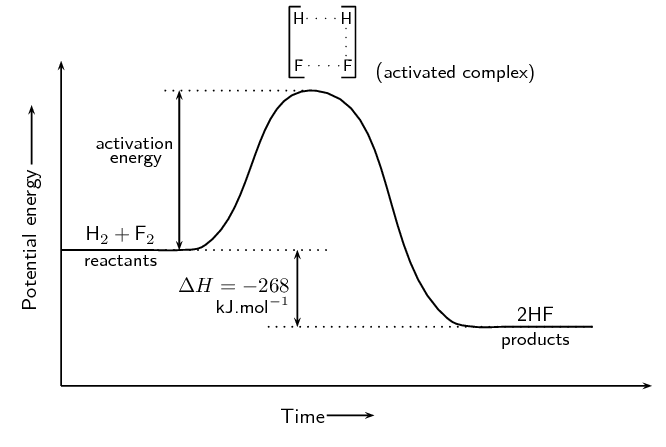
Progress of a Reaction: Key Components on a Potential Energy Diagram
- Reactants:
Located at the left of the graph, representing the starting materials of the reaction. - Activated Complex (Transition State):
The highest point on the graph, representing the most unstable state with the highest energy. - Products:
Found at the right of the graph, representing the final state after the reaction.
Activation Energy: The Energy Barrier
Activation Energy (Ea) is the energy required for reactants to reach the activated complex and convert to products. Think of it as the minimum energy necessary to “push” the reactants over the energy barrier.
- Lower Ea = Faster reaction (more likely to occur).
- Higher Ea = Slower reaction (less likely to occur).
Conceptual Understanding of Activation Energy
Activation energy is an “energy barrier” that reactants must overcome to initiate a reaction. The lower the barrier, the faster the reaction proceeds. On a PE diagram, Ea is represented as the difference in energy between the reactants and the transition state.
The Arrhenius Equation: Temperature and Reaction Rate
The Arrhenius equation describes how the rate constant (k) of a chemical reaction changes with temperature:
k=A⋅e(−RTEa)
- A: Frequency factor (related to the frequency of collisions).
- Ea: Activation energy.
- R: Universal gas constant.
- T: Temperature (in Kelvin).
Key Insight: The equation shows that as temperature increases, the rate constant and, consequently, the reaction rate also increase, due to more molecules having the minimum energy required for successful collisions.
Recap: Energy Changes in Chemical Reactions
- Endothermic vs. Exothermic: Reactions either absorb or release energy.
- Potential Energy Diagrams: Show energy changes throughout the reaction.
- Activation Energy: The minimum energy barrier reactants must overcome.
- Temperature Effects: Increasing temperature generally speeds up reactions due to more frequent and energetic collisions.