Atoms are the most basic unit of matter. When atoms interact, they form molecules. Depending on how their electrons interact, they may form either a covalent bond or an ionic bond.
In chemistry, everything strives to become as stable as possible. This means atoms bond to achieve a more stable, lower energy state. ⚖️
Principles of Bonding
Valence Electrons
The electrons that participate in bonding are the valence electrons—the outermost electrons in an atom. These electrons determine the chemical properties and bonding behavior of atoms. Let’s quickly review what we learned about valence electrons in Unit 1:
- Valence electrons are the outermost electrons in an atom.
- They are found in the s and p orbitals of the outermost occupied electron shell.
- A gap in ionization energies can indicate how many valence electrons an element has.
- Elements in the same group on the periodic table have the same number of valence electrons and thus form similar compounds with other elements.
Successive Ionization Energies in Kilojoules per Mole for the Elements in Period 3
| Element | I₁ | I₂ | I₃ | I₄ | I₅ | I₆ | I₇ |
|---|---|---|---|---|---|---|---|
| Na | 495 | 4560 | |||||
| Mg | 735 | 1445 | 7730 | ||||
| Al | 580 | 1815 | 2740 | 11,600 | |||
| Si | 780 | 1575 | 3220 | 4350 | 16,100 | ||
| P | 1060 | 1890 | 2905 | 4950 | 6270 | 21,200 | |
| S | 1005 | 2260 | 3375 | 4565 | 6950 | 8490 | 27,000 |
| Cl | 1255 | 2295 | 3850 | 5160 | 6560 | 9360 | 11,000 |
| Ar | 1527 | 2665 | 3945 | 5770 | 7230 | 8780 | 12,000 |
- General Decrease in ionization energy down the column
- General Increase in ionization energy across the row
- Sharp increase after core electrons are removed (indicated by steps)
This table shows the successive ionization energies of elements in period 3. Notice the jump in ionization energies, corresponding to the number of valence electrons each atom has.
Electronegativity
As discussed in Unit 1, electronegativity is one of the most important periodic trends to understand before learning about bonding:
- Electronegativity is a measure of an atom’s ability to attract electrons in a bond.
- Electronegativity increases across a period (from left to right) on the periodic table ➡️. This happens because the number of protons in the nucleus increases, strengthening the positive charge and enhancing its ability to attract electrons.
- Electronegativity decreases down a group on the periodic table ⬇️. As atomic radius increases, the distance between the nucleus and the valence electrons grows, weakening the attractive force.
✨ Coulomb’s Law
Coulomb’s Law helps measure the attraction between atoms in a bond. It states that the force of attraction depends on two key factors:
- Magnitude of charge: The greater the charge, the stronger the attraction.
- Distance between the nuclei: The closer the atoms are to each other, the stronger the attraction.
This relationship directly explains the trend of electronegativity. As atoms get larger (down a group), the distance between the nucleus and valence electrons increases, causing the attraction (and electronegativity) to decrease.
Ionic Bonding
Ionic bonds form when valence electrons are transferred from one atom to another, typically from a metal to a nonmetal.
Let’s look at an example of an ionic bond between sodium (Na) and chlorine (Cl):
Na(s)+21Cl2(g)→NaCl(s)
This forms NaCl, a brittle salt with a high melting point.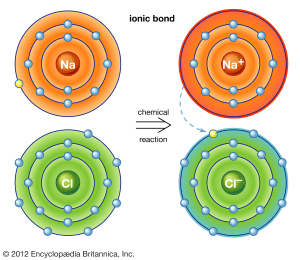
How Ionic Bonds Work:
Ionic bonds are held together by electrostatic forces between positive and negative ions. Unlike covalent bonds (where electrons are shared), ionic bonds form when one atom gives an electron to another. These forces are very strong, which is why ionic compounds tend to have high melting and boiling points.
- Crystal Lattice: Ionic compounds arrange themselves in a crystal lattice, a 3D structure where ions are held together by electrostatic forces. This structure explains why ionic compounds are rigid and have high melting points.
- Conductivity: Ionic compounds are good electrical conductors when melted or dissolved in water because the ions can move freely within the lattice, allowing them to conduct electricity. ⚡
Example: NaCl Formation
When sodium (Na) and chlorine (Cl) react, sodium loses a valence electron, and chlorine gains that electron. This process creates two ions:
- Sodium becomes a cation (Na⁺), with a positive charge.
- Chlorine becomes an anion (Cl⁻), with a negative charge.
These oppositely charged ions are held together by ionic bonds. The attraction between the positive and negative charges is explained by Coulomb’s Law.
Applying Coulomb’s Law: Ionic Bonds and Melting Points
According to Coulomb’s Law, higher charges and smaller distances between ions result in stronger attractions. This means that ionic compounds with larger charges or smaller ions have higher melting points.
Key Tip: When comparing the melting points of different ionic compounds, always look at the charge first. Compounds with higher ion charges will have stronger attractions and thus higher melting points.
Summary of Ionic Bonding:
- Ionic bonds form through the transfer of electrons between a metal and a nonmetal.
- These bonds are held together by strong electrostatic forces (explained by Coulomb’s Law).
- Ionic compounds typically have high melting/boiling points and form crystal lattices.
- Ionic compounds are good conductors when molten or dissolved in water.
For more details on valence electrons and bonding, make sure to check out our study guide on valence electrons and ionic compounds before diving into the next section!
Examples with Ionic Compounds and Melting Points
Which compound has a higher melting point: MgF₂ or NaF?
To determine which compound has a higher melting point, we look at ion charges first.
- Magnesium (Mg²⁺) has a +2 charge, while sodium (Na⁺) has a +1 charge.
- Since MgF₂ has a higher charge difference than NaF, MgF₂ has a higher melting point due to stronger electrostatic forces between ions.
Which compound has a higher melting point: LiF or NaBr?
When comparing LiF and NaBr, we see that both pairs of ions have the same charge (+1/-1), so we must consider ion size.
- Lithium (Li) and fluorine (F) are smaller ions compared to sodium (Na) and bromine (Br).
- Keeping in mind periodic trends, LiF has smaller ions, meaning LiF has a higher melting point than NaBr. This is because the smaller size leads to stronger electrostatic forces, requiring more energy to break.
Remember: As you move down a group on the periodic table, the atomic radius increases, which weakens the attraction between ions and lowers the melting point.
Covalent Bonding
Covalent bonds form when electrons are shared between two or more atoms, typically nonmetals. There are two types of covalent bonds depending on the electronegativity of the atoms involved:
- Polar covalent bond: Electrons are unequally shared, resulting in an unequal distribution of charge.
- Nonpolar covalent bond: Electrons are equally shared, resulting in a balanced distribution of charge. ⚖️
We will dive deeper into polarity when we discuss molecular geometry later in this unit.
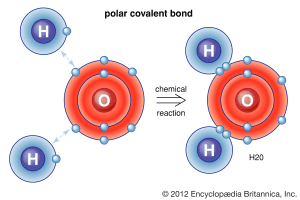
Example of a Polar Covalent Bond – H₂O
In a water molecule (H₂O), we see two O-H polar covalent bonds. Since oxygen has a much higher electronegativity than hydrogen, it pulls the shared electrons closer to itself, resulting in a partial negative charge (δ⁻) on the oxygen and a partial positive charge (δ⁺) on the hydrogen atoms.
This uneven electron distribution is what makes water polar. These strong polar attractions are crucial for water’s unique properties, making it essential for life on Earth. 🌍
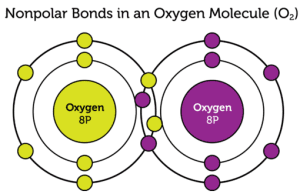
Example of a Nonpolar Covalent Bond – O₂
In a molecule of oxygen (O₂), the two oxygen atoms share electrons equally, as they have the same electronegativity.
This results in a nonpolar covalent bond, where the electron distribution is balanced. Since both atoms pull on the electrons with equal strength, there is no partial charge.
Distinguishing Between Nonpolar and Polar Covalent Bonds
To differentiate between polar and nonpolar covalent bonds, we must consider the electronegativity values of the atoms involved:
- In nonpolar covalent bonds, atoms with similar electronegativities share electrons equally.
- Example: In O₂, both oxygen atoms have the same electronegativity, so the electrons are shared evenly, making the bond nonpolar.
- In polar covalent bonds, atoms with unequal electronegativities share electrons unevenly.
- Example: In H₂O, oxygen has an electronegativity of 3.44, while hydrogen’s is 2.2. This difference causes oxygen to attract electrons more strongly, resulting in a partial negative charge on oxygen and a partial positive charge on hydrogen.
Key point: The greater the difference in electronegativity, the greater the polarity of the bond.
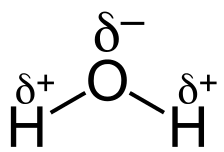
In diagrams, we use the Greek letter delta (δ) to represent partial charges.
Which Chemical Bond Will Form?
Here are some general guidelines to help you determine whether an ionic or covalent bond will form between two elements:
Ionic Bonds
- Form between elements with an electronegativity difference of greater than 1.7.
- Typically form between a metal and a nonmetal.
- Join a cation (positive ion) with an anion (negative ion).
Covalent Bonds
- Usually form between two nonmetals.
- Polar covalent bonds form when the electronegativity difference is between 0.4 and 1.7.
- Nonpolar covalent bonds form when the electronegativity difference is 0 to 0.4.
- Example: The C-H bond in methane (CH₄) has an electronegativity difference that falls within this range, making it nonpolar.
Characteristics of Ionic vs. Covalent Bonds
Ionic Compounds:
- High melting points
- Conduct electricity when dissolved in water (due to free-flowing ions)
- Good conductors of heat and electricity in molten or aqueous states
Covalent Compounds (Molecular):
- Low melting points
- Do not conduct electricity in any state
Network Solids:
- High melting points
- Do not conduct electricity even when molten or in solution
- Form from strong covalent bonds throughout the structure (e.g., diamond or silicon dioxide)