3.2 Types of Solids
In this unit, we’ve discussed intramolecular forces within ionic solids, metallic solids, and alloys. Now that we’ve learned about intermolecular forces (IMFs), let’s dive into the different types of solids.
The structures and properties of solids can be classified according to the forces that hold the atoms together:
- Ionic solids: Held together by electrostatic forces (Coulomb’s Law) between cations and anions.
- Metallic solids: Held together by metallic bonds.
- Covalent network solids: Held together by an extended network of covalent bonds (think diamonds💎).
- Molecular solids: Held together by weak IMFs.
Let’s explore each type in detail!
Amorphous Solids
Amorphous solids do not have a long-range, periodic crystal structure. Their structures are disordered due to rapid cooling. Examples of amorphous solids include gum, glass, and rubber. These solids won’t be studied in-depth for the AP Chemistry exam, but you should be aware of them.
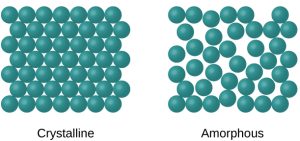
Crystalline Solids
Crystalline solids include the types of solids you need to know for the AP Chemistry exam: ionic solids, covalent network solids, molecular solids, and metallic solids. In crystalline solids, particles are arranged in a regularly repeating pattern, forming a crystal lattice.
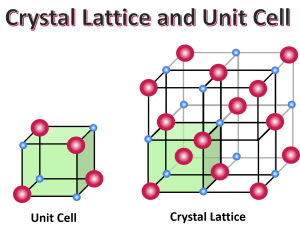
Structure of Crystalline Solids
The smallest repeating unit that makes up a crystal lattice is called a unit cell. A crystal lattice is formed by repeating these unit cells in all directions.
Metallic Solids
Metallic solids are composed of metal atoms held together by metallic bonds. When metals ionize, they lose their valence electrons and become positive ions (cations). Metallic bonding can be visualized as an array of cations surrounded by a “sea” of delocalized electrons.
Properties of metallic solids:
- Good conductors of electricity ⚡ (due to delocalized electrons)
- High melting and boiling points 🌡️ (due to strong bonds)
- Shiny appearance 🌟
- Malleability and ductility 🔌
👉 Want to learn more about metallic solids? Check out our discussion in Unit 2: 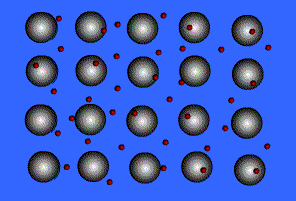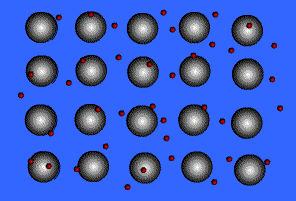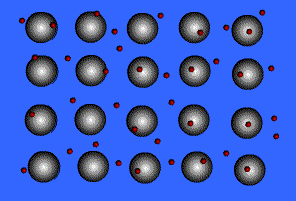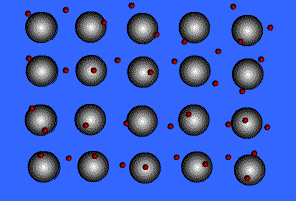
Alloys
Alloys are composed of two or more elements, with at least one being a metal. Alloys can be homogeneous or heterogeneous.
- Substitutional alloys: Atoms of the minority element substitute for atoms of the majority element in the crystal lattice.
- Interstitial alloys: Smaller atoms occupy spaces (“interstices”) between the larger atoms in the crystal structure.
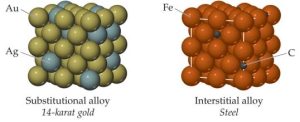
Ionic Solids
Ionic solids consist of cations and anions held together in a crystal lattice by electrostatic forces. Due to these strong interactions, ionic compounds typically have high melting points.
Key Trend:
- Stronger attractions occur when the charges of ions increase and/or the sizes of the ions decrease. (Coulomb’s Law)
Properties of ionic solids:
- Hard and brittle
- High melting and boiling points
- Poor conductors of electricity as solids, but good conductors when melted or dissolved (when ions are free to move).
Molecular Solids
Molecular solids are composed of atoms or molecules held together by IMFs. They have strong intramolecular forces (covalent bonds), but relatively weak IMFs between molecules.
Properties of molecular solids:
- Low melting and boiling points 🌡️: IMFs are weak, so less energy is needed to break them.
- Brittle and hard 💎: Molecular solids are brittle due to weak IMFs.
- Poor conductors of electricity and heat 🚫🔌: Valence electrons are tightly held within covalent bonds, preventing the flow of electrons.
Examples: Ice (H₂O) and sucrose (C₁₂H₂₂O₁₁)
Covalent Network Solids
Covalent network solids consist of atoms held together in large networks by covalent bonds. These solids are much harder and have higher melting points than molecular solids. Important examples include diamond and graphite, which are both made of carbon.
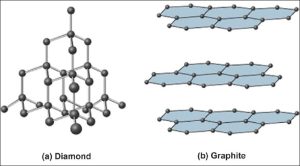
Graphite
- Layers of carbon atoms arranged in rings.
- sp² hybridized with delocalized electrons → good conductor of heat and electricity.
- Strong bonds within the layers, but weak bonds between the layers → soft, as layers can slide past each other.
Diamond
- Hardest naturally occurring substance.
- sp³ hybridized → very strong 3D network.
- Insulator.
Comparing Properties of Solids
Here’s a summary table comparing the different types of solids:
| Type of Solid | Form of Unit Particles | Forces Between Particles | Properties | Examples |
|---|---|---|---|---|
| Molecular 🧊 | Atoms or Molecules | LDFs, dipole-dipole, hydrogen bonding | Soft, low melting point, poor conductor | Argon, methane, sucrose, dry ice |
| Covalent Network 💎 | Atoms in a covalent network | Covalent Bonds | Very hard, high melting point, poor conductor | Diamond, quartz |
| Ionic 🧂 | Positive and Negative Ions | Electrostatic attractions | Hard, brittle, high melting point, poor conductor | NaCl (salt) |
| Metallic ✨ | Atoms | Metallic Bonds | Varying hardness, high melting points, good conductor, malleable, ductile | Cu, Fe, Al |







