9.7 Galvanic (Voltaic) and Electrolytic Cells Explained
Exploring Electrochemistry ⚡
Electrochemistry is a branch of chemistry that focuses on the relationship between redox reactions and electrical energy. Through this study, we can measure cell potentials, determine spontaneity, and even harness electrical energy to drive chemical reactions. Let’s dive into the fundamentals of galvanic and electrolytic cells, breaking down their structures, principles, and applications in detail.
Redox Reaction Recap
Before we jump in, here’s a quick refresher: redox reactions involve the transfer of electrons between species. The reducing agent loses electrons (oxidation), while the oxidizing agent gains electrons (reduction). A helpful acronym to remember is OIL RIG—Oxidation Is Loss, Reduction Is Gain.
Example of a Redox Reaction:
In this reaction:
- Copper (Cu) is oxidized from 0 to +2 oxidation state.
- Silver (Ag) is reduced from +1 to 0 oxidation state.
We can split this into half-reactions:
- Oxidation Half-Reaction:
- Reduction Half-Reaction:

Image From SciencePhoto
Understanding Cell Potentials and Reduction Potentials
In a redox reaction, electrons flow from the reducing agent to the oxidizing agent, generating an electromotive force (EMF), which we measure in volts. This is referred to as the cell potential (Ecell). A positive cell potential indicates a spontaneous reaction, while a negative value indicates a nonspontaneous reaction.
Example Calculation: Consider the reaction:
Half-reactions:
- Oxidation: (; becomes +0.76 V as oxidation)
- Reduction: ()
Total cell potential:
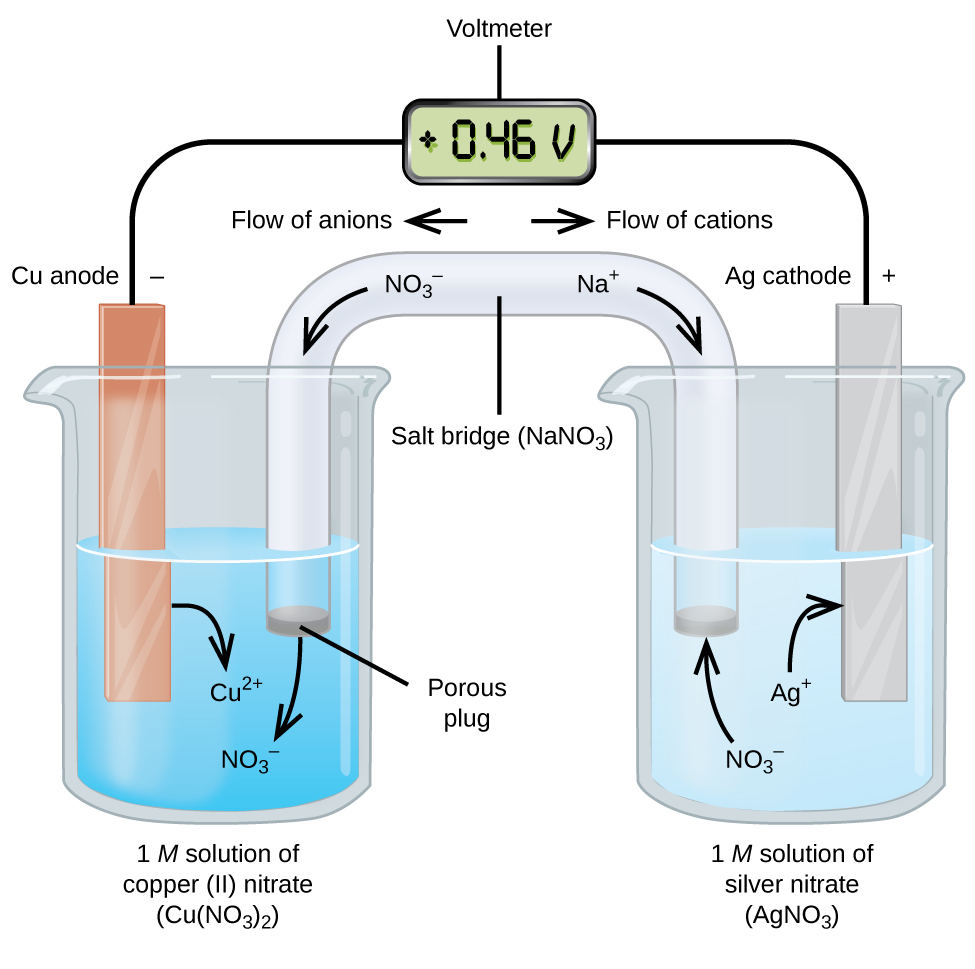
Galvanic (Voltaic) Cells: Spontaneous Reactions
A galvanic cell (or voltaic cell) uses spontaneous redox reactions to generate electrical energy. Here’s how it works:
- Oxidation occurs at the anode, releasing electrons.
- Reduction occurs at the cathode, gaining electrons.
- Electrons flow from the anode to the cathode through a wire, generating an electric current.
Example: Consider the reaction . Here:
- Anode: Copper (oxidation)
- Cathode: Silver (reduction)
- Electron Flow: From Cu (anode) to Ag (cathode)
The solutions are connected via a salt bridge (e.g., NaNO3) that maintains charge balance by allowing ion flow.
Electrolytic Cells: Driving Nonspontaneous Reactions
Electrolytic cells use electrical energy to drive nonspontaneous redox reactions:
- Example: Electrolysis of molten NaCl to produce Na and Cl2.
- Anode: Chlorine is oxidized to Cl2.
- Cathode: Sodium ions are reduced to Na.
The process requires external energy (e.g., a battery) to push electrons. Calculating Ecell confirms nonspontaneity:
Key Takeaways
- Galvanic cells generate energy from spontaneous reactions, while electrolytic cells require energy input to drive nonspontaneous reactions.
- Redox reactions drive electron flow, with oxidation at the anode and reduction at the cathode.
- Cell potential indicates spontaneity, with positive values for galvanic cells and negative for electrolytic cells.