9.4 Thermodynamic and Kinetic Control: Why Spontaneity Isn’t Always Fast
In the study of chemistry, there is often a misconception that thermodynamically favorable reactions happen quickly. The reality is more complex. Many spontaneous reactions progress at such a slow pace that they appear to remain unchanged, a phenomenon explained by kinetic control. This section explores the relationship between thermodynamics, kinetics, and why some reactions, despite being spontaneous, don’t proceed at an observable rate.
A Quick Review of Kinetics
Before diving into kinetic control, let’s briefly review key concepts from Unit 5. Kinetics focuses on the rate of a chemical reaction, essentially measuring how fast reactants turn into products. The reaction rate is defined as the change in reactant concentration over time:
Rate Laws
Reaction rates are also governed by rate laws:
In this equation, k is the rate constant, [A] and [B] are reactant concentrations, and n and m represent their reaction orders. A higher rate constant (k) and concentration mean a faster reaction.
Activation Energy (Ea)
To understand kinetic control, we must revisit activation energy (Ea), the minimum energy required for molecules to collide and react. Higher activation energy leads to slower reactions because fewer molecules have the necessary energy to overcome this barrier. The following diagram illustrates this concept:
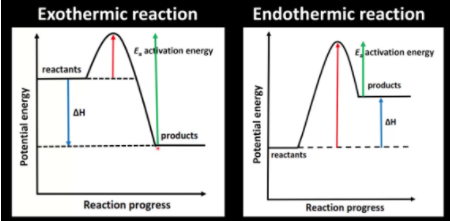
Image From Mike Sugiyama Jones
Thermodynamic Favorability and Kinetic Control
Gibbs Free Energy (ΔG°) tells us if a reaction is thermodynamically favorable (spontaneous) or unfavorable (nonspontaneous). A negative ΔG° indicates a spontaneous reaction, while a positive ΔG° signifies nonspontaneity. However, spontaneity does not equate to speed. Consider this real-world example:
Diamonds Turning to Graphite
The reaction Cdiamond(s) → Cgraphite(s) is spontaneous (ΔG° = -3 kJ). Yet, diamonds don’t visibly transform into graphite because the reaction rate is exceptionally slow—taking thousands of years due to high activation energy. This reaction exemplifies kinetic control, where a high activation energy barrier slows or prevents observable change.
Kinetically vs. Thermodynamically Controlled Reactions
- Kinetically Controlled: High activation energy slows the reaction, despite ΔG° suggesting spontaneity.
- Thermodynamically Controlled: Driven by Gibbs Free Energy differences; reaction behavior aligns with ΔG° predictions (spontaneous or nonspontaneous).
Why Reactions Fall Under Kinetic Control
The main reason for kinetic control is high activation energy. This energy barrier prevents otherwise favorable reactions from proceeding quickly. Fortunately, catalysts can lower the activation energy and speed up reactions. Catalysts work by altering the reaction mechanism, allowing reactions to occur at a measurable rate.
Example: Hydrogen Peroxide Decomposition
Hydrogen peroxide (H₂O₂) decomposition is slow under normal conditions. When a catalyst like iodide ions is introduced, it triggers the dramatic “Elephant’s Toothpaste” reaction, demonstrating how catalysts can shift reactions out of kinetic control.
Image From GIPHY
Overcoming Kinetic Barriers with Catalysts
Catalysts don’t change the thermodynamic favorability (ΔG°) of a reaction, but they make the reaction faster by lowering Ea. Imagine if there were a catalyst for Cdiamond(s) → Cgraphite(s); the transformation from diamond to graphite would occur at a measurable rate. This highlights the power of catalysis in overcoming kinetic hurdles.
Key Takeaways
- Thermodynamic favorability (ΔG° < 0) does not guarantee a fast reaction.
- Kinetic control arises from high activation energy, slowing reaction rates.
- Catalysts lower activation energy, increasing reaction speed without altering spontaneity.
Understanding the interplay between thermodynamics and kinetics is vital for predicting and controlling chemical behavior in both academic and industrial settings.
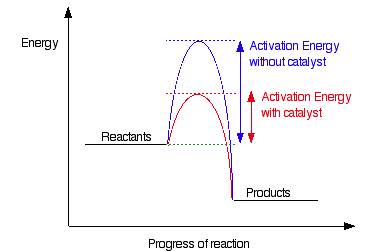
Image From Kerem’s Chemistry







