9.2 Absolute Entropy and Entropy Change in Chemistry
In our journey through thermodynamics, we now turn to entropy—a measure of the disorder or randomness in a system. Unlike enthalpy, which you encountered in Unit 5 and can only be expressed as a change (ΔH°), entropy can be represented in absolute terms (S°) or as a change (ΔS°). This post will explore both concepts and their significance in understanding chemical reactions.
Comparing S° (Absolute Entropy) and ΔS° (Change in Entropy)
Absolute Entropy (S°)
Absolute entropy quantifies the total disorder in a system at a given state under standard conditions (1 atm and 273 K). It reflects the possible arrangements or “chaos” present in a molecule. While calculating S° can be complex, you’ll typically be given these values for reactions on the AP Chemistry exam or find them listed in tables at the back of textbooks.
Key takeaway: S° values help compare different substances and are fundamental in understanding how much disorder a particular compound has inherently.
Change in Entropy (ΔS°)
ΔS° describes how entropy changes during a chemical reaction. Just as you applied Hess’s Law for enthalpy changes (ΔH°), you can use a similar method for entropy:
- ΔS° = Σ S° (products) – Σ S° (reactants)
Entropy is a state function, meaning its value is independent of the pathway taken to reach a final state. It only depends on the initial and final states. This concept ensures that ΔS° remains consistent, regardless of how a reaction occurs.
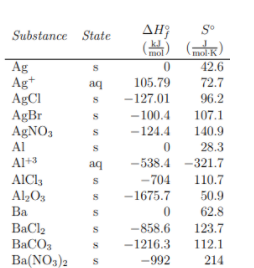
Image From Mr. Bigler
Understanding State Functions
A state function depends only on the initial and final states, not the path taken. Think of climbing a mountain: your altitude change remains constant whether you take a straight path or zigzag. In contrast, the distance traveled varies, making it a non-state function. Entropy, like altitude change, is a state function.
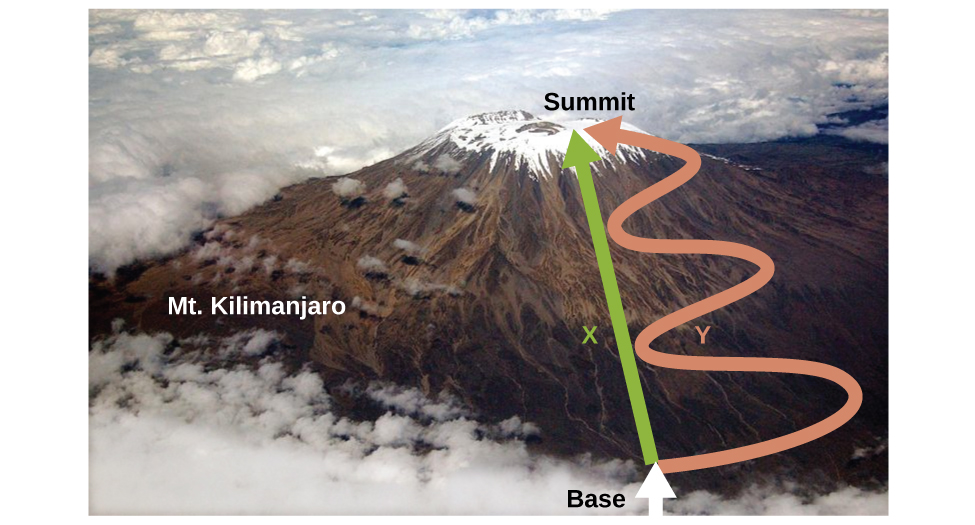
Image From Chemistry Stack Exchange
Applying the Entropy Change Formula
When calculating entropy changes, remember to consider stoichiometric coefficients! These values often appear as multipliers in the entropy calculation formula:
Interpreting ΔS°: Positive vs. Negative Values
The sign of ΔS° reveals whether a reaction becomes more or less ordered:
- Positive ΔS°: Indicates increased disorder (more randomness).
- Negative ΔS°: Indicates increased order (less randomness).
Example Problem 1: Calculating ΔS°
Given the following reaction and entropies of substances at 298 K:
- Reaction: 2KI (s) + Cl2 (g) → 2KCl (s) + I2 (s)
- S° values (J/mol•K): KI = 125, KCl = 150, Cl2 = 175, I2 = 250
Calculate ΔS°:
- Apply the formula:
ΔS° = Σ S° (products) – Σ S° (reactants)
ΔS° = [(2 * 150) + 250] – [(2 * 125) + 175]
ΔS° = (300 + 250) – (250 + 175)
ΔS° = 550 – 425 = -25 J/mol•K
Interpretation: The negative ΔS° indicates the system became more ordered during the reaction.
Example Problem 2: Predicting the Sign of ΔS°
Consider: 2Na (s) + Cl2 (g) → 2NaCl (s)
- Reactants: 3 total moles, including a gas (more disorder).
- Products: 2 moles of a solid (more order).
Since the reaction transitions from a more disordered state (with a gas) to a more ordered state (solid NaCl), ΔS° is negative, indicating a decrease in entropy.
Practical Implications of Entropy Changes
Understanding entropy changes helps chemists predict:
- Reaction spontaneity (combined with enthalpy and Gibbs Free Energy).
- Stability and feasibility of reactions.
- Energy dispersal in reactions, contributing to insights on reaction mechanisms.







