Solutions and Electrolytes
When analyzing chemical reactions, you’ll encounter the notation (s), (l), (g), or (aq) following compounds, which indicate their phase of matter. So far, we’ve focused on solids, liquids, and gases. Now, let’s dive into solutions, specifically aqueous solutions, where a substance is dissolved in water.
Types of Solutions
Solutions in which water acts as the dissolving medium, or solvent, are referred to as aqueous solutions
- Electrolytes: Substances whose aqueous solutions contain ions and can conduct electricity.
- Nonelectrolytes: Substances whose aqueous solutions contain no ions and cannot conduct electricity.
Electrolytes
Electrolytes are compounds that, when dissolved in water, form ions, allowing the solution to conduct electricity. Electrolytes can be strong or weak:
Strong Electrolytes:
- Strong electrolytes completely dissociate into their constituent ions in water. This means that when these substances dissolve, all of their molecules split into cations and anions.
- Examples:
- Soluble salts: NaCl, KCl.
- Strong acids: HCl, HBr, HNO₃.
- Strong bases: NaOH, Ca(OH)₂.
Weak Electrolytes:
- Weak electrolytes only partially dissociate in water, producing fewer ions than strong electrolytes. They conduct electricity, but not as well.
- Example: Acetic acid (CH₃COOH). When it dissolves in water, only some of the molecules split into hydrogen ions and acetate ions.
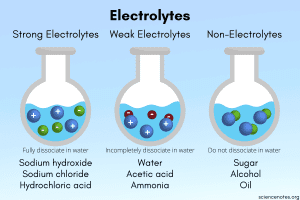
Nonelectrolytes:
Nonelectrolytes do not dissociate into ions at all. As a result, they do not conduct electricity.
- Example: Sugar (C₆H₁₂O₆) dissolved in water.
Image: Strong, weak, and nonelectrolyte dissociation comparison
Acids and Bases
Acids and bases are key electrolytes.
- Acids: Proton donors that increase the concentration of H⁺ (aq) in a solution.
- Bases: Proton acceptors that increase the concentration of OH⁻ (aq) in a solution.
Strong Acids:
These completely dissociate in water, releasing H⁺ ions.
- Examples include HCl, HNO₃, and H₂SO₄.
Strong Bases:
These dissociate completely to form OH⁻ ions.
- Examples include NaOH and KOH.
Colligative Properties
Colligative properties depend on the concentration of solute particles, not their identity. These properties include vapor pressure lowering, boiling point elevation, and freezing point depression.
Vapor-Pressure Lowering
When a non-volatile solute is added to a solvent, the vapor pressure of the solution decreases. This is because solute particles block solvent molecules from escaping into the gas phase, lowering the overall vapor pressure. This is quantified using Raoult’s Law:
Where:
- P1 = vapor pressure of the solution,
- Xs = mole fraction of the solvent,
- P0 = vapor pressure of the pure solvent.
Boiling-Point Elevation
When a solute is added to a solvent, the boiling point of the solution increases. This is because the vapor pressure is lowered, requiring a higher temperature to achieve boiling. The relationship is described by the formula:
Where:
- = change in boiling point,
- = ebullioscopic constant (a property of the solvent),
- = molality of the solution.
Freezing-Point Depression
Adding a solute to a solvent also lowers the freezing point. This is why salt is used to melt ice on roads in the winter. The freezing-point depression is described by the equation:
Where:
- = change in freezing point,
- = cryoscopic constant (a property of the solvent),
- = molality of the solution.
Image: Freezing-point depression illustrated with salt and ice
Summary
In this unit, we explored:
- Electrolytes (strong and weak) and nonelectrolytes.
- Acids and bases as electrolytes.
- Colligative properties, including vapor-pressure lowering, boiling-point elevation, and freezing-point depression.







