Molecular and Ionic Compound Structure and Properties
Now that we’ve covered the types of bonds, intramolecular forces, and the structure of ionic solids, it’s time to discuss metals and alloys!
Metallic Bonding
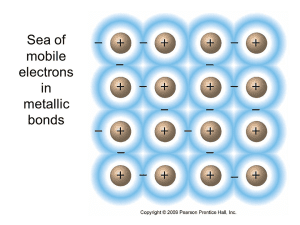
While ionic and covalent substances are commonly discussed, you should also be familiar with metallic substances and how they are structured. Metals have a lattice of cations surrounded by a “sea” of delocalized valence electrons.
Sea of Electrons
In metallic bonding:
- Metals ionize by losing valence electrons, becoming positive ions (cations).
- The valence electrons move freely throughout the entire structure, forming a “sea” around the metal cations.
This means that in metallic substances:
- The nucleus and core electrons stay in place, while the valence electrons are mobile and don’t belong to any specific atom. These delocalized electrons are responsible for many unique properties of metals.
Properties of Metals
Good conductors of electricity: The delocalized electrons in metals are mobile, allowing metals to carry electric current.
High melting and boiling points: The metallic bonds are very strong, requiring significant energy to break them. Imagine trying to boil a metal like gold or iron!
Shiny appearance: The reflection of light from delocalized electrons gives metals their characteristic shiny look.
Malleability and ductility: Metals can be deformed without breaking (malleability) and can be stretched into wires (ductility) because their structure is not as rigid as ionic solids.
Comparing Solids
Here’s a comparison chart to help you distinguish between different types of solids:
| Type of Solid | Form of Unit Particles | Forces Between Particles | Properties | Examples |
|---|---|---|---|---|
Molecular  | Atoms or Molecules | LDFs, dipole-dipole, hydrogen bonds | Fairly soft, low melting point, bad conductor | Argon, methane, sucrose, dry ice |
Covalent-Network  | Atoms connected in a covalent network | Covalent bonds | Very hard, very high melting point, bad conductor | Diamond, quartz |
Ionic  | Positive and negative ions | Electrostatic attractions | Hard, brittle, high melting point, bad conductor | NaCl (salt), KBr |
Metallic  | Atoms | Metallic bonds | Varying hardness, high melting points, good conductor, malleable, ductile | Cu, Fe, Al |
(Table courtesy of an unknown source)
For now, you should focus on ionic and metallic solids, as the others will be covered in Unit 3 when we dive deeper into intermolecular forces.
Alloys
Metals can also bond with other elements to create alloys. Alloys are formed when two or more elements (with at least one being a metal) are mixed in their liquid form and then cooled to create a solid alloy. The properties of the alloy depend on the specific ratio of substances used.
There are two types of alloys you should know:
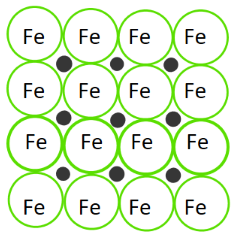
 Interstitial Alloys
Interstitial Alloys
- Form when smaller atoms fit into the spaces between larger metal atoms.
- Example: Steel is an alloy of iron and carbon. Iron forms the base, and carbon fills in the spaces between iron atoms due to its smaller atomic radius.
- Properties: Strength and hardness due to the added small atoms, making the structure more dense.
 Substitutional Alloys
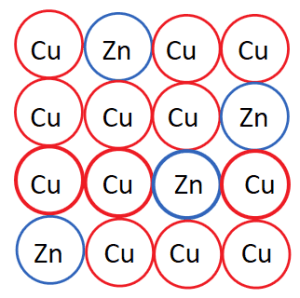
Substitutional Alloys
- Form when atoms of similar size replace one another in the metal structure.
- Example: Brass, an alloy of copper and zinc, where zinc atoms substitute copper atoms in the lattice.
- Properties: Good electrical and thermal conductivity due to the presence of delocalized electrons.
Comparing Interstitial and Substitutional Alloys
- Interstitial alloys add smaller atoms to the structure (e.g., steel), making them harder and denser.
- Substitutional alloys involve the replacement of atoms of similar size (e.g., brass), often improving conductivity.

Check Your Understanding
Let’s look at this practice question:
(1) A student tested two solids—Fe (iron) and FeCl₂ (iron chloride)—for their ability to conduct electricity. Here are the results:
| Solids | Does it conduct electricity? |
|---|---|
| Fe (s) | Yes |
| FeCl₂ (s) | No |
(a) Explain the results the student observed.
(b) Could the FeCl₂ sample conduct electricity under different conditions?
Sample Responses:
For part (a):
- Iron (Fe) conducted electricity because it is a metal. Metals have delocalized valence electrons (the “sea of electrons”), which allows them to conduct electricity.
- FeCl₂ did not conduct electricity because it is an ionic solid. In its solid form, the ions are fixed in a lattice, and the electrons are not free to move.
For part (b):
- Melt the FeCl₂: In its liquid form, the ions would be free to move, allowing it to conduct electricity.
- Dissolve FeCl₂ in water: When dissolved, the ions become mobile in the aqueous solution, allowing electrical conductivity.









