What Is Urea? Everything You Need to Know
Imagine a world where the very essence of life’s recycling process and the cornerstone of modern agriculture converge in one simple chemical compound. Did you know that urea is not only a waste product of protein metabolism in humans and animals but also the most widely used nitrogen fertilizer in the world? This dual nature of urea—both as a natural metabolic byproduct and a critical industrial chemical—raises an intriguing question: what is urea? In this comprehensive guide, we’ll explore everything you need to know about urea—from its definition and key properties to its historical discovery, real-world applications, and modern relevance. Whether you’re a biology student, an agricultural professional, a chemistry enthusiast, or simply curious about how this fascinating molecule impacts our everyday lives, this article will provide you with an in-depth, reader-friendly understanding of what is urea and why it matters.
Table of Contents
- Introduction: The Dual Nature of Urea
- What Is Urea? A Clear and Concise Definition
- Key Characteristics and Properties of Urea
- Historical and Contextual Background
- In-Depth Exploration of Urea
- Real-World Examples and Case Studies
- The Importance, Applications, and Benefits of Urea
- Addressing Common Misconceptions and FAQs
- Modern Relevance and Current Trends
- Conclusion: Celebrating the Multifaceted Role of Urea
- Additional Resources for Further Exploration
1. Introduction: The Dual Nature of Urea
Have you ever stopped to consider that a single compound can be both a byproduct of life and a driver of modern industry? Urea is a striking example of this duality. Produced naturally by the body as a waste product from the breakdown of proteins, urea is also synthesized on a massive scale for use in agriculture, where it plays a crucial role in enhancing crop yields. This fascinating chemical, with its seemingly simple structure, holds tremendous significance across biological, industrial, and environmental domains.
In this article, we will answer the question: what is urea? We will embark on an exploration that covers:
- A straightforward definition of urea and its chemical nature.
- The key properties and characteristics that define urea.
- A historical overview tracing urea’s discovery and evolution as a concept.
- In-depth discussions on the roles of urea in biological systems, its industrial synthesis, and its pivotal applications in agriculture and medicine.
- Real-world examples and case studies that illustrate the practical impact of urea.
- The significance and benefits of understanding urea for scientific, economic, and environmental progress.
- Common misconceptions and FAQs to clear up any misunderstandings.
- Modern trends and current research that continue to shape our understanding of urea.
By the end of this guide, you’ll not only understand what is urea but also appreciate its vital role in sustaining life and driving technological innovation.
2. What Is Urea? A Clear and Concise Definition
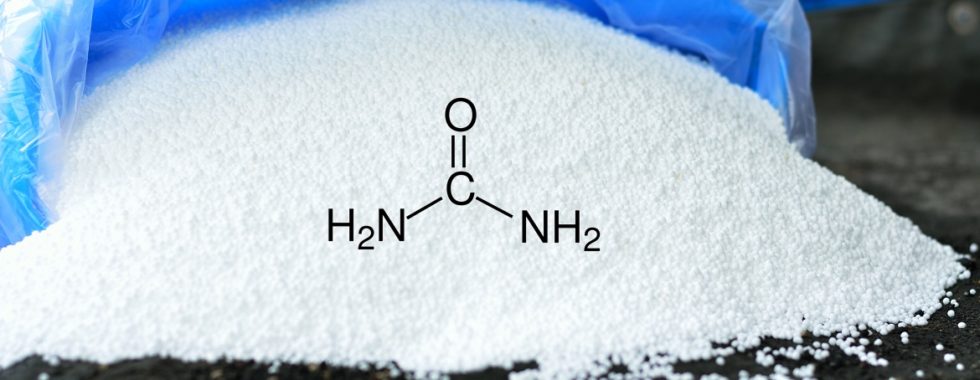
So, what is urea? Urea is a simple organic compound with the chemical formula CO(NH₂)₂, also known as carbamide. It is produced naturally in the liver as a way to remove excess nitrogen resulting from the breakdown of proteins. This compound is then excreted by the kidneys in urine. In industrial settings, urea is synthesized through chemical processes and is primarily used as a nitrogen-rich fertilizer.
Breaking Down the Definition
Chemical Composition:
Urea consists of one carbonyl group (CO) flanked by two amine groups (NH₂). This structure gives urea its unique properties, making it highly soluble in water and an effective source of nitrogen.Biological Role:
In living organisms, urea is the main end product of protein metabolism. It plays a critical role in the urea cycle, a metabolic pathway that converts toxic ammonia into urea for safe excretion.Industrial Importance:
Urea is one of the most widely produced chemicals globally. Its high nitrogen content makes it a valuable fertilizer, supporting global agriculture by promoting plant growth and increasing crop yields.
In summary, what is urea? It is both a natural metabolic waste product and a vital industrial chemical, bridging the realms of biology and agriculture with its unique chemical properties and versatile applications.
3. Key Characteristics and Properties of Urea
Understanding what is urea requires an exploration of its core characteristics and properties, which explain its behavior in both biological and industrial contexts.
Physical Properties
- Appearance:
Urea typically appears as a white, crystalline solid. - Solubility:
It is highly soluble in water, which is crucial for its role in biological systems and as a fertilizer. - Melting Point:
Urea has a melting point of approximately 132.7°C (271°F), making it stable under normal conditions. - Chemical Stability:
It is relatively stable chemically but can decompose under high temperatures to produce ammonia and isocyanic acid.
Chemical Properties
- Nitrogen Content:
Urea is valued for its high nitrogen content, which is essential for plant growth. - Reactivity:
The molecule can undergo hydrolysis, breaking down into ammonia and carbon dioxide—a reaction that is fundamental to its use as a fertilizer. - Versatility:
Urea’s chemical structure allows it to be used as a building block in the synthesis of plastics, resins, adhesives, and pharmaceuticals.
Biological Properties
- Role in the Urea Cycle:
Urea is the primary means by which mammals excrete excess nitrogen. The urea cycle converts ammonia, a toxic byproduct of protein metabolism, into urea, which is less toxic and easily eliminated. - Indicator of Metabolic Function:
The concentration of urea in the blood and urine is an important marker of kidney function and overall metabolic health.
These properties underscore the dual importance of urea in both biological and industrial applications, offering a window into what is urea and why it is a compound of immense significance.
4. Historical and Contextual Background
The story of urea is a fascinating chapter in the history of science—a journey that spans from its natural role in metabolism to its groundbreaking synthesis in the laboratory.
The Discovery of Urea
Robert Hooke and Early Observations:
The term “cell” was coined by Hooke in the 17th century, but urea itself began to capture the attention of scientists in the 18th and 19th centuries as researchers delved into the chemistry of living organisms.Friedrich Wöhler’s Breakthrough:
In 1828, German chemist Friedrich Wöhler made one of the most significant discoveries in organic chemistry by synthesizing urea from inorganic compounds. This breakthrough shattered the long-held belief in vitalism—the idea that organic compounds could only be produced by living organisms—and paved the way for modern organic chemistry.
Milestones in Urea Research
Advancements in Synthesis:
Wöhler’s synthesis of urea is often considered the birth of organic chemistry. Since then, the industrial production of urea has been refined and scaled up, making it one of the most produced chemicals globally.Role in Agriculture:
The development of urea as a nitrogen fertilizer revolutionized agriculture. Its widespread use has significantly increased crop yields and contributed to food security around the world.Medical and Industrial Applications:
Over time, research has expanded urea’s applications beyond agriculture. It is now used in pharmaceuticals, skincare products, and industrial processes, underscoring its versatility and importance.
Notable Historical Anecdotes
Wöhler’s Synthesis and Its Impact:
Friedrich Wöhler’s unexpected synthesis of urea from ammonium cyanate is a landmark moment in scientific history. This discovery not only debunked vitalism but also opened the door to the systematic study and synthesis of organic compounds.Agricultural Transformation:
The adoption of urea fertilizer in the 20th century is a key example of how a scientific discovery can have a profound impact on global agriculture, transforming economies and improving lives.
These historical insights provide essential context for understanding what is urea and its transformative impact on science, agriculture, and industry.
5. In-Depth Exploration of Urea
To truly grasp what is urea, we must examine its various dimensions—from its biological role and industrial synthesis to its applications in agriculture, medicine, and beyond.
A. Urea in Biological Systems
1. The Urea Cycle
- Function:
In mammals, the urea cycle is a critical metabolic pathway that converts toxic ammonia (a byproduct of protein metabolism) into urea. This urea is then transported to the kidneys for excretion. - Significance:
This process is essential for detoxifying the body and maintaining a safe level of nitrogen. Without it, the accumulation of ammonia could be lethal. - Key Enzymes:
The cycle involves several enzymes, including carbamoyl phosphate synthetase, ornithine transcarbamylase, and arginase, each playing a specific role in the conversion process.
2. Urea as a Waste Product
- Excretion:
In humans and other animals, urea is excreted in urine. It is a primary indicator of kidney function and overall metabolic health. - Clinical Significance:
Elevated urea levels in the blood (uremia) can signal kidney dysfunction, while low levels might indicate malnutrition or liver disease.
B. Industrial Production and Chemical Synthesis of Urea
1. The Chemical Synthesis Process
- Haber-Bosch Process:
Modern industrial production of urea begins with the synthesis of ammonia via the Haber-Bosch process. Ammonia is then combined with carbon dioxide under high pressure and temperature to form urea. - Reaction Conditions:
The reaction is typically carried out under conditions that favor the formation of urea, with catalysts used to increase efficiency and yield. - Economic Impact:
Urea is one of the most produced chemicals globally due to its critical role in agriculture, making its production economically significant for many countries.
2. Environmental Considerations
- Energy Consumption:
The industrial synthesis of urea is energy-intensive. Advances in process engineering are continuously being made to improve energy efficiency and reduce the environmental footprint. - Emission Controls:
Innovations such as carbon capture and alternative energy sources are being explored to mitigate the environmental impact of urea production.
C. Urea in Agriculture: The Fertilizer Revolution
1. The Role of Urea as a Nitrogen Fertilizer
- Nutrient Supply:
Urea is a rich source of nitrogen, a vital nutrient that supports plant growth, protein synthesis, and overall crop yield. - Application:
Farmers widely use urea as a fertilizer to enhance soil fertility and boost agricultural productivity. Its high nitrogen content makes it an effective means of replenishing depleted soils.
2. Economic and Global Impact
- Agricultural Productivity:
The use of urea has dramatically increased crop yields worldwide, playing a pivotal role in feeding a growing global population. - Cost-Effectiveness:
Urea is relatively inexpensive and easy to transport, making it a popular choice among farmers. - Challenges:
Despite its benefits, excessive use of urea can lead to environmental issues such as soil degradation and water pollution. Sustainable practices and slow-release formulations are being developed to address these challenges.
D. Urea in Medicine and Pharmaceuticals
1. Medical Applications of Urea
- Dermatology:
Urea is commonly used in skincare products due to its moisturizing and keratolytic properties. It helps treat conditions like dry skin, eczema, and psoriasis by softening and breaking down rough, scaly skin. - Pharmaceuticals:
Urea is also used as an excipient in various drug formulations. Its ability to solubilize certain compounds and enhance drug delivery makes it a valuable component in pharmaceutical manufacturing.
2. Clinical Importance
- Diagnostic Marker:
The concentration of urea in blood and urine is an important diagnostic marker for kidney function and overall metabolic health. Regular monitoring of urea levels can help in the early detection and management of kidney disorders. - Research Applications:
Urea is used in laboratory settings as a protein denaturant and in electrophoresis techniques. Its role in scientific research contributes to advances in biochemistry and molecular biology.
E. Other Industrial and Commercial Applications
1. Chemical Industry and Manufacturing
- Resins and Plastics:
Urea is a key component in the production of urea-formaldehyde resins, which are used in adhesives, particle boards, and laminates. These resins are valued for their strength and durability. - Agricultural Chemicals:
Beyond fertilizers, urea is used in the formulation of pesticides and herbicides, contributing to the broader field of agricultural chemistry.
2. Environmental and Energy Applications
- Diesel Exhaust Fluid (DEF):
Urea is a critical component in Diesel Exhaust Fluid, which is used in selective catalytic reduction (SCR) systems to reduce harmful emissions from diesel engines. This application highlights urea’s role in environmental protection and sustainable energy practices. - Water Treatment:
Urea can be used in water treatment processes to remove contaminants and improve water quality, further demonstrating its versatility.
6. Real-World Examples and Case Studies
Real-world examples and case studies provide concrete illustrations of what is urea and how it functions across various domains.
A. Case Study: Urea as a Fertilizer in Agriculture
Background:
Urea fertilizer is a cornerstone of modern agriculture. Its high nitrogen content makes it a critical resource for boosting crop yields and maintaining soil fertility.
Key Points:
- Application Techniques:
Farmers apply urea to fields in various forms, from granular applications to liquid formulations. Advances in fertilizer technology, such as slow-release urea, aim to optimize nutrient uptake and reduce environmental impact. - Economic Impact:
The widespread use of urea has significantly increased global food production, contributing to food security and economic stability in many regions. - Challenges and Solutions:
Overuse of urea can lead to soil acidification and water contamination. Sustainable practices, including precision agriculture and balanced fertilization, are being implemented to mitigate these issues.
B. Case Study: Urea in Diesel Exhaust Fluid for Emission Reduction
Background:
Environmental regulations have necessitated the reduction of harmful emissions from diesel engines. Urea, as a component of Diesel Exhaust Fluid (DEF), plays a key role in this process.
Key Points:
- How DEF Works:
DEF is injected into the exhaust stream of diesel engines. In the presence of a catalyst, urea decomposes to form ammonia, which reacts with nitrogen oxides (NOₓ) to form harmless nitrogen and water. - Benefits:
This process significantly reduces air pollution, contributing to cleaner air and compliance with environmental standards. - Industry Adoption:
DEF has become standard in the automotive industry, especially in commercial transportation, where reducing emissions is critical for both regulatory compliance and public health.
C. Real-World Application: Urea in Medical and Research Laboratories
Example:
In biochemistry labs, urea is used as a protein denaturant in electrophoresis and other analytical techniques. It helps scientists study the structure and function of proteins by disrupting their three-dimensional configuration.
Impact:
- Advancing Research:
The use of urea in research has led to significant discoveries in protein chemistry and molecular biology. - Clinical Diagnostics:
Monitoring urea levels in patients’ blood and urine is essential for diagnosing kidney function and other metabolic conditions.
These examples and case studies underscore the multifaceted nature of what is urea and demonstrate its vital role across different sectors of society.
7. The Importance, Applications, and Benefits of Urea
Understanding what is urea is crucial because of its broad-ranging impact on both biological and industrial processes. Here are some of the key reasons why urea is so important:
A. Foundations for Medicine and Biotechnology
- Health Diagnostics:
Urea levels are a critical marker in assessing kidney function and overall metabolic health. Regular monitoring can aid in the early detection of renal diseases and metabolic imbalances. - Pharmaceutical Applications:
Urea is used in the formulation of various medications and skincare products. Its ability to interact with proteins makes it a valuable component in drug delivery systems and dermatological treatments. - Regenerative Medicine:
Research into urea’s role in cellular metabolism contributes to advancements in tissue engineering and regenerative therapies.
B. Economic and Agricultural Impact
- Global Food Production:
As a primary nitrogen fertilizer, urea plays an indispensable role in modern agriculture. Its application has revolutionized crop production, ensuring higher yields and contributing to food security worldwide. - Cost-Effectiveness:
Urea is relatively inexpensive to produce and transport, making it accessible to farmers across the globe. - Environmental Considerations:
While urea has enormous benefits in agriculture, its overuse can lead to environmental challenges such as water pollution and soil degradation. Ongoing research aims to optimize its use, balancing productivity with sustainability.
C. Industrial and Environmental Applications
- Chemical Synthesis:
Urea serves as a precursor in the manufacture of resins, plastics, adhesives, and various chemical compounds, underpinning numerous industrial processes. - Emission Control:
Its application in Diesel Exhaust Fluid (DEF) is pivotal in reducing harmful emissions from diesel engines, playing a key role in environmental protection and sustainable energy practices. - Water Treatment and Beyond:
Urea is also used in certain water treatment processes, further highlighting its versatility and importance in various environmental and industrial contexts.
D. Educational and Research Benefits
- Scientific Literacy:
Studying urea and its applications provides foundational knowledge in biochemistry, organic chemistry, and environmental science, enhancing overall scientific literacy. - Research and Innovation:
Urea is a focal point in numerous research projects aimed at improving agricultural practices, developing new pharmaceuticals, and creating sustainable industrial processes.
By understanding what is urea, you gain valuable insights into one of the most essential compounds in both nature and industry—a compound that underpins everything from human health to global food production.
8. Addressing Common Misconceptions and FAQs
Despite its widespread use and importance, several misconceptions about what is urea persist. Let’s address these misunderstandings and answer some frequently asked questions.
Debunking Myths About Urea
Myth 1: Urea Is Only a Waste Product
Reality:
Although urea is produced in the body as a way to excrete excess nitrogen, its significance extends far beyond being a mere waste product. It is a crucial component in nitrogen metabolism and has extensive industrial applications.Myth 2: Urea Is Dangerous or Toxic
Reality:
Urea is generally safe when handled properly. It is a naturally occurring substance in the body and is used in a variety of consumer products, including fertilizers and skincare products. However, like any chemical, it must be managed responsibly in industrial settings.Myth 3: All Urea Is the Same
Reality:
There are different grades and formulations of urea, each designed for specific applications. Agricultural urea, industrial-grade urea, and pharmaceutical-grade urea differ in purity and intended use.Myth 4: Urea’s Only Role Is in Agriculture
Reality:
While urea is a cornerstone of modern agriculture, its applications span medicine, industry, environmental science, and research. Its versatility makes it an indispensable chemical in many fields.
Frequently Asked Questions (FAQs)
Q: What is urea?
A: Urea is an organic compound with the chemical formula CO(NH₂)₂. It is a key byproduct of protein metabolism in animals and is also synthesized industrially for use in fertilizers, pharmaceuticals, and various chemical processes.Q: How is urea produced in the body?
A: Urea is produced in the liver through the urea cycle, which converts toxic ammonia, a byproduct of protein breakdown, into urea, which is then excreted by the kidneys.Q: What are the main industrial applications of urea?
A: Urea is widely used as a nitrogen fertilizer in agriculture. It is also a precursor for the manufacture of resins, plastics, adhesives, and is used in Diesel Exhaust Fluid (DEF) for emission control.Q: Why is urea important for agriculture?
A: Urea is an excellent source of nitrogen, which is essential for plant growth. Its high nitrogen content and cost-effectiveness make it a critical fertilizer for boosting crop yields and ensuring food security.Q: Are there environmental concerns related to urea use?
A: Overuse of urea as a fertilizer can lead to environmental issues such as water pollution and soil degradation. However, sustainable practices and innovative formulations are being developed to minimize these risks.
9. Modern Relevance and Current Trends in Urea Research
The study and application of what is urea continue to evolve with technological advancements and new scientific discoveries. Here, we explore some modern trends and current debates related to urea.
A. Innovations in Urea Production and Use
Energy-Efficient Production:
Advances in industrial processes are making the production of urea more energy-efficient and environmentally friendly. Researchers are exploring catalysts and alternative energy sources to reduce the carbon footprint of urea synthesis.Enhanced Fertilizer Formulations:
The development of slow-release and controlled-release urea formulations aims to optimize nutrient uptake by plants, reducing environmental impact and increasing agricultural productivity.
B. Urea in Environmental Technology
Emission Control:
Urea’s role in Diesel Exhaust Fluid (DEF) is expanding as governments worldwide implement stricter emission standards. Innovations in DEF technology are critical for reducing harmful pollutants from diesel engines.Water and Soil Remediation:
Research is underway to explore the use of urea in environmental remediation, particularly in improving soil fertility and managing agricultural runoff.
C. Urea in Biotechnology and Medicine
Medical and Pharmaceutical Applications:
Urea is finding new applications in dermatology, where its moisturizing and keratolytic properties are used in treating skin conditions such as eczema and psoriasis. In pharmaceuticals, urea is used as a reagent and stabilizer in drug formulations.Stem Cell and Regenerative Research:
Emerging studies are investigating the role of urea in cellular metabolism and tissue regeneration, with potential implications for regenerative medicine and personalized therapies.
D. Future Directions
Interdisciplinary Research:
The future of urea research lies at the intersection of chemistry, biology, environmental science, and engineering. Interdisciplinary approaches will likely yield new insights into improving urea production, reducing environmental impact, and expanding its applications.Global Impact:
As the global population grows and food security becomes an even more pressing issue, innovations in urea use and sustainability will be crucial for supporting agriculture and reducing the environmental footprint of fertilizer use.
These modern trends underscore that what is urea is not a static concept but a dynamic field of study with far-reaching implications for health, industry, and the environment.
10. Conclusion: Celebrating the Multifaceted Role of Urea
In our comprehensive exploration of what is urea, we have journeyed through the remarkable story of a compound that is fundamental to life and modern industry. Urea is much more than a mere waste product; it is a versatile chemical that plays critical roles in biology, agriculture, medicine, and technology.
Key Takeaways
Definition and Essence:
Urea is an organic compound with the formula CO(NH₂)₂. It is produced naturally as a byproduct of protein metabolism and is also synthesized industrially for a wide range of applications.Core Properties:
Urea’s high solubility in water, rich nitrogen content, and chemical stability make it invaluable in biological systems and industrial processes.Historical Significance:
Friedrich Wöhler’s groundbreaking synthesis of urea marked the beginning of modern organic chemistry, challenging the notion of vitalism and paving the way for future scientific discoveries.Real-World Impact:
Urea plays a vital role in agriculture as a fertilizer, in medicine as a component of skincare and pharmaceuticals, and in industry as a building block for resins, plastics, and emission control systems.Modern Relevance:
Ongoing research and technological innovations continue to expand our understanding of what is urea and enhance its applications, ensuring its importance in the 21st century and beyond.
A Call to Action
Understanding what is urea empowers you to appreciate one of the most essential compounds in both nature and industry. We invite you to:
- Explore Further:
Delve into additional resources, academic courses, and research articles to deepen your understanding of cell metabolism, organic chemistry, and agricultural sciences. - Engage in Discussions:
Share your insights, questions, or experiences with urea—whether in academic settings, industry, or everyday life—with friends, colleagues, or online communities. - Apply the Knowledge:
Use your understanding of urea to make informed decisions about topics ranging from environmental sustainability and agricultural practices to personal health and industrial innovation. - Spread the Word:
Share this article with anyone interested in exploring the fascinating world of urea and its far-reaching impact on our lives.
Your journey into understanding what is urea not only enriches your knowledge but also contributes to a broader appreciation of the science that sustains life and drives progress.
11. Additional Resources for Further Exploration
For those eager to further explore the captivating subject of urea, here are some reputable resources:
Websites and Online Articles:
- National Institutes of Health (NIH) – Find research articles and detailed information on urea’s role in human metabolism and kidney function.
- PubMed Central – Access a wealth of peer-reviewed scientific literature on urea and related metabolic processes.
- United States Department of Agriculture (USDA) – Explore information on the use of urea as a fertilizer and its impact on agriculture.
Books:
- “Organic Chemistry” by Paula Yurkanis Bruice – A comprehensive text that covers the chemistry of urea and its synthesis.
- “Biochemistry” by Jeremy M. Berg, John L. Tymoczko, and Lubert Stryer – Offers detailed insights into metabolic pathways, including the urea cycle.
- “Fertilizer Manual” by the International Fertilizer Association – Provides practical information on urea fertilizer, its applications, and environmental considerations.
Educational Platforms:
- Khan Academy – Biology and Chemistry:
Visit Khan Academy for accessible tutorials on metabolic processes and organic chemistry, including sections on urea. - Coursera and edX:
Look for courses on biochemistry, environmental science, and agricultural technology that cover the applications and significance of urea.
- Khan Academy – Biology and Chemistry:
Academic Journals:
- Journal of Agricultural and Food Chemistry – Publishes research on fertilizers, including the use and impact of urea in agriculture.
- Biochemical Journal – Features articles on metabolic pathways and the biochemistry of nitrogen compounds, including urea.
Final Thoughts
Understanding what is urea is a journey into the heart of life’s chemical processes and industrial innovation. From its role as a natural byproduct of protein metabolism to its pivotal position as a globally significant fertilizer and industrial chemical, urea is a compound that embodies the intricate connections between biology, chemistry, and our modern way of life. By exploring its definition, properties, historical evolution, and diverse applications, we gain not only a deeper appreciation for this remarkable compound but also insights into the broader systems that sustain life and drive progress.
Thank you for joining us on this in-depth exploration of urea. We hope this guide has provided you with valuable insights and a comprehensive understanding of what is urea and its transformative impact on health, agriculture, industry, and the environment. Please share your thoughts, questions, and experiences in the comments below—your engagement enriches our discussion and helps build a community of curious, informed minds.


 4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all
4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all



