“What is Surface Tension” – Everything You Need to Know
Surface tension is a fascinating natural phenomenon that plays a critical role in everyday life, science, and technology. In this comprehensive guide, we’ll dive deep into understanding what is surface tension, its origins, characteristics, and how it affects the world around us. Whether you’re a student, educator, or curious reader, this post is designed to answer all your questions and expand your knowledge.
Introduction: The Invisible Force Shaping Our World
Have you ever noticed how water droplets bead up on a freshly waxed car or how some insects can walk on water? These curious observations are a direct result of surface tension. This blog post unpacks the concept by exploring:
- The Definition: What exactly is surface tension?
- The Science Behind It: How do molecules interact at the surface?
- Historical Context: Key milestones in understanding surface tension.
- Real-World Applications: From everyday phenomena to advanced scientific research.
- Common Misconceptions: Debunking myths and answering frequently asked questions.
- Modern Relevance: Recent developments and ongoing research in the field.
By the end of this post, you’ll have a well-rounded understanding of what is surface tension and why it is an essential aspect of both natural processes and technological innovations.
What is Surface Tension? A Straightforward Definition
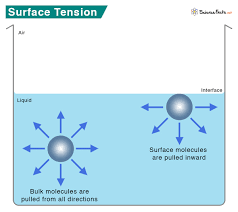
At its core, surface tension is a property of liquids that arises from unbalanced molecular cohesive forces at or near the surface. To put it simply, the molecules at the surface of a liquid are pulled inward by neighboring molecules, creating a “skin” that resists external force. This phenomenon is most observable in water due to its strong hydrogen bonding, though it is present in many other liquids as well.
Key Characteristics of Surface Tension
- Molecular Cohesion: Surface tension is driven by cohesive forces—attractive forces between similar molecules. In water, these forces are primarily hydrogen bonds.
- Minimization of Surface Area: Liquids naturally adopt shapes that minimize surface area, such as the spherical shape of droplets, due to surface tension.
- Interface Behavior: The surface tension is particularly noticeable at the interface between two phases (e.g., water and air), where there is an imbalance of forces.
- Temperature Dependence: Surface tension decreases with an increase in temperature as molecular motion disrupts cohesive interactions.
Historical and Contextual Background
Understanding what is surface tension has evolved over centuries. The exploration of surface tension dates back to early scientific observations and experiments.
Early Discoveries and Scientific Milestones
- 17th and 18th Centuries: Early scientists like Galileo Galilei and Robert Hooke made initial observations regarding the behavior of liquids, setting the stage for later discoveries.
- 18th Century – Pioneering Experiments: Physicists such as Isaac Newton and later, the French scientist Pierre-Simon Laplace, developed theories related to capillary action and the curvature of liquid surfaces.
- 19th Century – The Rise of Molecular Science: The advent of molecular theory allowed scientists to start understanding the forces at play on a microscopic level. Thomas Young and Pierre-Simon Laplace further formalized the mathematical description of surface tension, leading to the well-known Young-Laplace equation.
- 20th Century – Technological Applications: With the industrial revolution and advancements in materials science, the principles of surface tension found applications in fields like inkjet printing, microfluidics, and even the design of new materials with special wetting properties.
Notable Anecdotes and Events
- The Water Strider Mystery: One of the most captivating illustrations of surface tension is the ability of water striders to walk on water. This phenomenon puzzled early naturalists and eventually contributed to a deeper understanding of liquid surfaces.
- The Bubble Experiment: Early experiments with soap bubbles provided visual and measurable evidence of surface tension. These experiments not only highlighted the minimization of surface area but also showcased the dynamic behavior of molecules at the surface.
In-Depth Exploration: Unpacking the Science Behind Surface Tension
To fully grasp what is surface tension, we must examine the fundamental scientific principles that govern it. This section delves into the mechanics, molecular interactions, and physical implications of surface tension.
Molecular Interactions: The Heart of Surface Tension
Cohesive vs. Adhesive Forces
- Cohesive Forces: These are the forces that attract like molecules to each other. In liquids, cohesive forces create a net inward pull on the molecules at the surface, leading to a contraction of the surface.
- Adhesive Forces: These are the forces that attract dissimilar molecules to each other. When a liquid comes into contact with a solid, adhesive forces determine how well the liquid spreads or wets the surface.
The balance and competition between these forces explain why some liquids spread out on a surface while others form droplets.
The Role of Hydrogen Bonding in Water
Water is often the go-to example when discussing surface tension due to its unique molecular structure. Each water molecule can form up to four hydrogen bonds with neighboring molecules, resulting in a high degree of cohesion. This is why water exhibits a relatively high surface tension compared to many other liquids.
The Physics Behind the “Skin” of a Liquid
The Concept of Minimizing Energy
The phenomenon of surface tension is fundamentally an energy minimization process. Molecules at the surface of a liquid are in a higher energy state because they are not fully surrounded by other molecules. To reduce this energy, the liquid minimizes its surface area.
- Spherical Droplets: A sphere has the smallest possible surface area for a given volume, which is why droplets tend to form spherical shapes.
- Capillary Action: In narrow tubes, surface tension can cause liquids to rise or fall depending on the balance between cohesive and adhesive forces.
The Young-Laplace Equation
A critical tool in understanding surface tension is the Young-Laplace equation, which mathematically describes the pressure difference across the curved surface of a liquid. The equation is given by:
where:
- is the pressure difference across the surface,
- is the surface tension,
- and are the radii of curvature in two perpendicular directions.
This equation explains why smaller droplets have higher internal pressure compared to larger droplets and helps predict behaviors in complex fluid systems.
Observing Surface Tension in Action
Everyday Examples
- Water Droplets on Leaves: When raindrops land on a leaf, they often form near-perfect spheres due to the minimization of surface area by surface tension.
- Soap Bubbles: The vibrant, iridescent films of soap bubbles are classic examples. Soap reduces the surface tension of water, allowing bubbles to form and persist.
Industrial and Technological Applications
- Inkjet Printing: Modern inkjet printers rely on precise control of surface tension to eject tiny droplets of ink onto paper.
- Microfluidics: In lab-on-a-chip devices, the manipulation of small volumes of fluids is achieved by exploiting surface tension, which governs fluid behavior at micro scales.
- Cleaning and Coating: Detergents and surfactants reduce surface tension, making it easier to remove dirt and grime from surfaces. This principle is widely used in cleaning products and industrial processes.
Importance, Applications, and Benefits of Understanding Surface Tension
Understanding what is surface tension is not merely an academic pursuit; it has real-world implications across numerous fields. Here, we explore its significance in everyday life, industrial applications, and cutting-edge scientific research.
Everyday Life
The Wonders of Nature
- Insect Locomotion: Many insects, such as water striders, exploit surface tension to move across water surfaces without sinking.
- Formation of Dew and Rain: Surface tension influences how water droplets form in the atmosphere, impacting weather patterns and precipitation.
Household Applications
- Cleaning: Knowledge of surface tension helps in formulating effective detergents and cleaning agents. Surfactants, which reduce surface tension, enable water to spread more easily and penetrate fabrics and surfaces.
- Cooking: Chefs and food scientists use the principles of surface tension in culinary techniques, such as creating foams, emulsions, and gels.
Scientific and Technological Applications
Material Science
- Coatings and Films: Engineers design advanced coatings and films by controlling surface tension, which affects adhesion, wetting, and spreading properties.
- Nanotechnology: At the nanoscale, surface forces dominate over gravitational forces. Understanding surface tension is crucial for developing nanoscale devices and materials.
Environmental Science
- Pollution Control: Surface tension plays a role in the dispersion and breakdown of oil spills. Surfactants are used to mitigate environmental disasters by promoting the formation of smaller droplets, which can be more easily biodegraded.
- Water Treatment: In water purification systems, controlling surface tension can enhance the removal of contaminants and improve the efficiency of filtration processes.
Biomedical Applications
- Drug Delivery: The development of micro- and nano-sized drug delivery systems often relies on manipulating surface tension to control the formation of particles and capsules.
- Diagnostics: Surface tension principles are applied in lab-on-a-chip technologies that enable rapid diagnostic testing and disease detection.
Economic and Industrial Benefits
- Enhanced Product Design: By understanding and controlling surface tension, manufacturers can design products with improved performance, whether it’s a more effective adhesive, a longer-lasting coating, or an innovative microfluidic device.
- Cost Efficiency: Optimizing surface tension in industrial processes can lead to significant cost savings by reducing waste, improving material usage, and increasing process efficiency.
- Innovation and Research: Continued research into surface tension drives innovation across multiple industries, paving the way for new technologies and applications that benefit society as a whole.
Common Misconceptions and FAQs About Surface Tension
Despite its fundamental role in both natural phenomena and technological applications, there are several misconceptions about surface tension. Let’s address some frequently asked questions to clarify these misunderstandings.
FAQs: Clearing Up the Confusion
Q1: Is surface tension the same as viscosity?
A: No, surface tension and viscosity are different properties of liquids. Viscosity is a measure of a liquid’s resistance to flow, while surface tension refers to the cohesive force at the liquid’s surface.
Q2: Does surface tension only affect water?
A: Although water is a classic example due to its strong hydrogen bonds, surface tension is a property inherent to all liquids. The magnitude of the surface tension depends on the specific intermolecular forces within the liquid.
Q3: Can surface tension be completely eliminated?
A: Surface tension can be significantly reduced by adding surfactants or detergents, but it cannot be entirely eliminated as it is a fundamental property arising from molecular interactions.
Q4: Why do insects like water striders not break the water surface?
A: Water striders have lightweight bodies and specially adapted legs that distribute their weight evenly. The surface tension of water creates a film strong enough to support them without breaking.
Q5: How does temperature affect surface tension?
A: Generally, as temperature increases, surface tension decreases. Higher temperatures increase molecular motion, reducing the cohesive forces at the surface.
Q6: What role does surface tension play in the formation of droplets and bubbles?
A: Surface tension is the driving force behind the formation of spherical droplets and bubbles. It minimizes the surface area for a given volume, leading to the formation of shapes that have the lowest possible energy state.
Addressing Other Common Myths
Myth: Surface tension is an optical illusion or a property that only exists in laboratory conditions.
- Fact: Surface tension is a measurable, physical property that has profound effects in nature and technology. It is as real and impactful as gravity.
Myth: Adding any substance to water will always decrease its surface tension.
- Fact: While many additives (like detergents) decrease surface tension, some substances can increase it. The specific effect depends on the molecular interactions between the additive and water.
Modern Relevance and Current Trends in Surface Tension Research
The study of surface tension remains an active field of research with significant implications for modern science and industry. Recent developments are pushing the boundaries of how we understand and manipulate this phenomenon.
Advances in Measurement Techniques
- High-Precision Instruments: New technologies, such as atomic force microscopy (AFM) and advanced tensiometers, allow scientists to measure surface tension with unprecedented precision.
- Real-Time Monitoring: Developments in imaging and spectroscopy have enabled real-time monitoring of surface dynamics at the microscopic level, shedding light on transient behaviors and interactions.
Innovations in Materials Science
- Smart Surfaces: Researchers are designing smart surfaces that can dynamically alter their surface tension properties in response to external stimuli such as light, temperature, or pH changes.
- Self-Cleaning Materials: Inspired by natural systems (like lotus leaves), scientists are developing materials that mimic these surfaces by exploiting controlled surface tension and micro/nanostructures.
Environmental and Biomedical Research
- Enhanced Oil Recovery: Innovations in reducing surface tension are playing a key role in enhanced oil recovery techniques, where surfactants are used to mobilize trapped oil in reservoirs.
- Targeted Drug Delivery: Understanding surface tension at the nanoscale is critical for the development of targeted drug delivery systems that rely on controlled encapsulation and release of therapeutic agents.
Nanotechnology and Microfluidics
- Lab-on-a-Chip Devices: In microfluidic systems, precise control over surface tension is crucial for manipulating tiny volumes of fluids. This is revolutionizing fields such as medical diagnostics, environmental monitoring, and chemical synthesis.
- Self-Assembly Processes: Surface tension-driven self-assembly is being harnessed to create complex nanostructures with potential applications in electronics, photonics, and materials engineering.
Current Research Debates
- Interfacial Phenomena in Complex Fluids: Scientists are actively researching how surface tension behaves in non-Newtonian fluids, emulsions, and biological systems, which often exhibit more complex interactions.
- Molecular Dynamics Simulations: With the rise of computational power, researchers are using molecular dynamics simulations to better predict and understand the behavior of surface tension at the atomic level.
These trends highlight that what is surface tension is not just a static property but an evolving area of study with far-reaching implications.
Conclusion: Embracing the Complexity and Beauty of Surface Tension
In this comprehensive exploration, we have answered the question, what is surface tension, by breaking down its definition, historical background, molecular mechanics, and diverse applications. From the formation of water droplets and soap bubbles to advanced industrial processes and cutting-edge research, surface tension is a pivotal concept that bridges everyday experiences with complex scientific phenomena.
Key Takeaways
- Definition and Properties: Surface tension is the result of cohesive molecular forces that create a “skin” at the surface of a liquid.
- Historical Context: The understanding of surface tension has evolved from early observations to modern scientific theories and applications.
- Real-World Applications: Surface tension affects a wide range of phenomena, from natural events like rain and insect locomotion to technological advancements in printing, coatings, and biomedical devices.
- Modern Relevance: Advances in measurement techniques, materials science, and nanotechnology continue to push the boundaries of what we know about surface tension.
Call to Action
We encourage you to explore further and share your thoughts on how understanding surface tension can impact other fields. Whether you are a student, researcher, or simply curious, the world of surface phenomena offers endless opportunities for discovery. If you found this post insightful, please share it with others and join the conversation in the comments below. What other natural phenomena intrigue you as much as surface tension?
Additional Resources and Links
For those looking to dive even deeper into the subject, here are some reputable sources and further readings:
- Scientific Journals: Explore journals like Langmuir and The Journal of Physical Chemistry for cutting-edge research articles on interfacial phenomena.
- Educational Websites: Websites like Khan Academy and the American Chemical Society offer accessible explanations and interactive modules related to surface tension.
- Books: Consider reading texts such as Intermolecular and Surface Forces by Jacob Israelachvili, which provides an in-depth look at the physics behind these phenomena.
Final Thoughts
Understanding what is surface tension enriches our comprehension of the physical world. From the intricate dance of water molecules to the development of innovative technologies, surface tension is a subtle yet powerful force that shapes our daily experiences and drives scientific progress.
As you reflect on this post, consider the following:
- Next time you see a droplet of water or a soap bubble, remember the complex interplay of forces that make these phenomena possible.
- Think about how innovations in controlling surface tension might revolutionize the way we design materials and devices in the future.
- Share this knowledge with peers, students, and anyone curious about the hidden forces that govern the behavior of liquids.
Thank you for joining us on this deep dive into what is surface tension. Stay curious, and keep exploring the wonders of science and nature!


 4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all
4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all



