“What is Specific Gravity” Everything You Need to Know: Understanding the Measure of Density
Have you ever wondered why a ship floats on water while a rock sinks, or why the consistency of a liquid matters in industrial processes? The answer to these everyday curiosities lies in a key physical property known as specific gravity. In this comprehensive guide, we’ll explore what is specific gravity—its definition, origins, and importance. We’ll break down its essential characteristics, examine how it is measured, and explore its diverse applications in fields ranging from engineering and manufacturing to geology and environmental science. Whether you’re a student, a professional, or simply a curious mind, this article will equip you with the insights you need to understand specific gravity and appreciate its critical role in our world.
Introduction: The Hidden Measure Behind Floating and Sinking
Imagine a world where you could predict with precision whether an object will float or sink simply by knowing one number. Specific gravity is that number—a dimensionless value that reveals a material’s density relative to a reference substance, usually water. This concept not only underpins many natural phenomena (like why ice floats on water) but also plays a vital role in industries ranging from oil exploration to quality control in manufacturing.
Did you know?
Specific gravity is so fundamental that it is used in designing everything from ships and airplanes to high-performance batteries and even brewing the perfect beer! Understanding what is specific gravity can help you make informed decisions, improve product quality, and solve practical problems in everyday life.
In this article, we will cover:
- A clear, concise definition of specific gravity.
- The essential characteristics and properties that define specific gravity.
- The historical evolution of the concept and key milestones in its development.
- In-depth exploration of measurement techniques and real-world applications.
- The significance and benefits of understanding specific gravity in various fields.
- Common misconceptions and FAQs that clarify misunderstandings.
- Modern relevance and current trends, including new research and technological innovations.
By the end of this post, you’ll have a comprehensive understanding of what is specific gravity and why this seemingly simple measure is so powerful in science, industry, and everyday life. Let’s dive into the fascinating world of specific gravity.
What is Specific Gravity? A Straightforward Definition
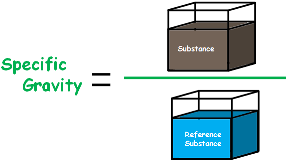
Specific gravity is defined as the ratio of the density of a substance to the density of a reference substance, usually water at 4°C (39°F) for liquids and solids, and air for gases. It is a dimensionless number, meaning it has no units, and it provides a relative measure of how dense a material is compared to the reference.
Essential Characteristics of Specific Gravity
Dimensionless Ratio:
Since specific gravity is a ratio, it is expressed as a number without units. For example, if a substance has a specific gravity of 2, it is twice as dense as the reference substance.Relative Density:
Specific gravity directly compares the density of a substance to that of a standard (water for most materials). This comparison helps simplify the analysis of materials in various contexts.Temperature Sensitivity:
Because density can vary with temperature, the reference temperature is critical. For liquids and solids, water at 4°C is used because it is the point of maximum density for water.Indicator of Material Properties:
Specific gravity provides insights into the composition and behavior of a material, influencing buoyancy, material selection, and process design.
Understanding these characteristics is key to grasping what is specific gravity and its practical implications in multiple fields.
Historical and Contextual Background
The concept of specific gravity has evolved alongside the study of density and material properties. Its development reflects a long history of scientific inquiry and practical application.
Early Origins in Science
Ancient Observations:
The basic idea of comparing the weight of substances can be traced back to ancient civilizations. Greek philosophers, including Archimedes, made early observations about buoyancy and density. Archimedes’ principle, which explains why objects float or sink, laid the groundwork for later studies in density and specific gravity.Medieval and Renaissance Developments:
As scientific methods advanced during the Renaissance, scholars began to quantify material properties more precisely. Early experiments with scales and balances allowed scientists to compare the densities of different materials, leading to a better understanding of relative density.
Modern Evolution and Standardization
19th Century Advances:
The 19th century saw the formalization of many scientific measurements. With the advent of modern chemistry and physics, the concept of specific gravity was refined and standardized. Researchers began to use specific gravity as a key parameter in characterizing substances, particularly in the burgeoning field of material science.Industrial Applications:
In the Industrial Revolution, specific gravity became crucial for industries such as mining, metallurgy, and brewing. For example, brewers used specific gravity to monitor fermentation and determine alcohol content, while miners used it to separate valuable ores from waste rock.Global Standards:
Today, specific gravity is a fundamental concept taught in science and engineering curricula around the world. It is standardized in scientific literature and industry practices, ensuring that measurements are consistent and comparable across different fields.
Notable Historical Anecdotes
Archimedes and the Crown:
One of the most famous historical anecdotes related to density involves Archimedes. Tasked with determining whether a crown was made of pure gold, Archimedes reportedly discovered the principle of buoyancy while taking a bath. His method—comparing the displacement of water—laid the conceptual foundation for understanding specific gravity.Brewing Revolution:
In the brewing industry, the development of the hydrometer in the 18th century revolutionized the process of fermentation monitoring. Brewers could now measure the specific gravity of wort and beer, enabling them to produce consistent, high-quality brews and paving the way for modern industrial brewing techniques.
These historical insights illustrate that what is specific gravity is a concept with deep roots in scientific discovery and practical innovation.
In-Depth Exploration: Key Components, Measurement, and Applications of Specific Gravity
To fully understand what is specific gravity, we need to examine its components, how it is measured, and its wide-ranging applications across different fields.
1. Components and Measurement of Specific Gravity
a. Understanding Density
Density:
Density is defined as mass per unit volume, typically expressed in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). It is a measure of how much matter is packed into a given space.Relation to Specific Gravity:
Specific gravity is the ratio of a substance’s density to the density of a reference substance (usually water for solids and liquids). The formula is:This ratio provides a relative measure of density that is easy to interpret.
b. Measurement Techniques
Hydrometer:
A hydrometer is a device used to measure the specific gravity of liquids. It floats in the liquid, and the level at which it floats indicates the density relative to water.Pycnometer:
A pycnometer is a specialized flask used to measure the density of a liquid accurately. It allows for precise calculation of specific gravity by comparing the weight of a known volume of liquid to the weight of the same volume of water.Digital Density Meters:
Modern digital instruments can measure density with high precision, automatically calculating specific gravity and providing data for quality control and research.
c. Factors Affecting Specific Gravity
Temperature:
Because the density of water changes with temperature, specific gravity measurements are typically standardized at 4°C for accuracy.Impurities:
The presence of dissolved solids or impurities in a liquid can affect its density and, consequently, its specific gravity.
2. Applications of Specific Gravity
Specific gravity is used in various fields, each benefiting from its ability to provide a quick, relative measure of density.
a. Industrial and Engineering Applications
Quality Control:
In industries such as manufacturing, mining, and food processing, specific gravity is used to ensure the consistency and quality of products. For example, specific gravity tests can help determine the purity of metals or the concentration of solutions.Material Selection:
Engineers use specific gravity to choose materials for construction, manufacturing, and design. A material’s specific gravity can indicate its strength, durability, and suitability for specific applications.Fluid Mechanics:
In fluid mechanics, specific gravity is critical for designing systems such as pipelines, pumps, and hydraulic systems, where the density of a fluid affects its flow characteristics and energy requirements.
b. Environmental and Geological Applications
Water Quality and Pollution Control:
Specific gravity measurements can help monitor water quality by detecting changes in density caused by pollutants or dissolved substances.Geological Surveys:
In geology, specific gravity is used to identify minerals and assess the composition of rocks. It is a fundamental property that helps classify materials and predict their behavior in different environments.
c. Scientific and Research Applications
Laboratory Analysis:
In scientific research, specific gravity is used to characterize liquids, solids, and even biological samples. It is essential in chemistry for determining concentrations and in biology for assessing cell density.Pharmaceuticals:
The pharmaceutical industry relies on specific gravity measurements to ensure the correct formulation of drugs and to monitor the consistency of liquid medications.
d. Everyday Applications
Beverage Industry:
In brewing and winemaking, specific gravity is used to monitor fermentation and determine alcohol content. It provides a simple way to assess the progress of fermentation and the quality of the final product.Consumer Products:
Specific gravity is also a factor in everyday products like cosmetics, where the density of ingredients can affect texture, stability, and performance.
3. Real-World Examples and Case Studies
a. Industrial Quality Control
Case Study: Metal Purity in Manufacturing
A manufacturing company uses pycnometers to measure the specific gravity of molten metals during production. By ensuring that the specific gravity remains within a narrow range, the company maintains high-quality standards and minimizes defects in its final products.Impact:
Consistent specific gravity measurements help reduce waste, optimize material usage, and ensure that products meet safety and quality standards.
b. Environmental Monitoring
Case Study: Assessing Water Quality in a River
Environmental scientists regularly measure the specific gravity of river water to monitor changes in pollution levels. An unexpected increase in specific gravity might indicate a high concentration of dissolved minerals or pollutants, prompting further investigation.Impact:
These measurements enable early detection of environmental issues, allowing for timely remediation efforts to protect water quality and public health.
c. Beverage Production
Case Study: Fermentation in a Brewery
A craft brewery uses hydrometers to measure the specific gravity of its beer during fermentation. This data helps brewers determine when fermentation is complete and estimate the alcohol content of the beer.Impact:
Accurate specific gravity readings ensure product consistency, quality, and optimal flavor, enhancing customer satisfaction and brand reputation.
d. Geological Analysis
Case Study: Mineral Identification
Geologists in a mining company use specific gravity tests to differentiate between valuable ore and waste rock. By measuring the specific gravity of rock samples, they can quickly identify high-value deposits.Impact:
This technique improves the efficiency of the mining process, reduces costs, and minimizes environmental disruption by targeting only the most promising areas.
Importance, Applications, and Benefits of Understanding Specific Gravity
Understanding what is specific gravity is crucial for several reasons, and its applications extend across multiple domains:
1. Enhancing Scientific and Engineering Precision
Accurate Material Characterization:
Specific gravity provides a simple yet powerful tool for characterizing materials, ensuring that engineers and scientists have reliable data for designing structures, processes, and products.Optimizing Performance:
In industries ranging from aerospace to automotive manufacturing, knowing the specific gravity of materials helps optimize performance, improve efficiency, and ensure safety.
2. Driving Environmental and Economic Sustainability
Resource Management:
Specific gravity measurements are vital in managing natural resources, assessing water quality, and monitoring pollution levels, thereby supporting environmental sustainability.Economic Decision-Making:
For industries such as mining and manufacturing, precise specific gravity data can drive cost savings, reduce waste, and improve the quality of products, contributing to overall economic growth.
3. Informing Research and Innovation
Foundational Science:
In academic and industrial research, specific gravity is a fundamental parameter that underpins experiments, product development, and scientific discovery.Technological Advances:
Innovations in sensor technology, digital measurement tools, and data analytics are enhancing our ability to measure and utilize specific gravity, driving progress in multiple fields.
4. Everyday Applications
Consumer Awareness:
For consumers, understanding specific gravity can improve decision-making when purchasing products that rely on material density, such as cosmetics, food products, and household appliances.Quality of Life:
From the beverages we enjoy to the safety of the vehicles we drive, specific gravity impacts everyday life in countless ways.
Addressing Common Misconceptions and FAQs about Specific Gravity
Even though specific gravity is a fundamental concept in science and engineering, several misconceptions persist. Let’s address these misunderstandings and provide clear answers to frequently asked questions.
Common Misconceptions
Misconception 1: Specific Gravity is the Same as Density
Reality:
While related, density is the mass per unit volume of a material, whereas specific gravity is the ratio of the density of a material to the density of a reference substance (usually water for solids and liquids). Specific gravity is dimensionless and provides a relative measure.Misconception 2: Temperature Doesn’t Affect Specific Gravity
Reality:
Since the density of water changes with temperature, specific gravity measurements are typically standardized at 4°C (39°F) for liquids and solids. Temperature variations can affect the reading if not properly controlled.Misconception 3: Specific Gravity is Only Relevant in Scientific Research
Reality:
Specific gravity has practical applications in various industries, including brewing, mining, manufacturing, and even consumer product quality control.
Frequently Asked Questions (FAQs)
Q: What exactly is specific gravity?
A:
Specific gravity is the ratio of the density of a substance to the density of a reference substance (commonly water at 4°C), expressed as a dimensionless number.Q: How is specific gravity measured?
A:
Specific gravity is measured using instruments such as hydrometers, pycnometers, or digital density meters. These tools compare the density of a sample to that of the reference substance.Q: Why is water at 4°C used as the reference temperature?
A:
Water is most dense at 4°C, making it a stable and reliable reference point for measuring the density of other substances.Q: How does specific gravity impact everyday products?
A:
Specific gravity influences the quality and performance of products—from ensuring the proper consistency of beverages to determining the strength and durability of construction materials.Q: Can specific gravity be used to assess purity?
A:
Yes. In industries like mining and brewing, specific gravity measurements are used to assess the purity and composition of materials, helping to determine quality and consistency.
Modern Relevance and Current Trends in Specific Gravity
In our technology-driven world, the concept of what is specific gravity continues to evolve with advancements in measurement techniques, digital technology, and sustainable practices. Here are some current trends and developments:
1. Digital Sensor Technology and Automation
- High-Precision Digital Sensors:
Modern digital density meters and hydrometers provide highly accurate specific gravity measurements, essential for quality control in industries like pharmaceuticals, food production, and aerospace. - Integration with IoT:
The Internet of Things (IoT) has enabled real-time monitoring of specific gravity in industrial processes, ensuring consistent quality and immediate feedback for process optimization.
2. Environmental and Sustainable Applications
- Water Quality Monitoring:
Specific gravity is a critical parameter in environmental science. Advanced monitoring systems use specific gravity measurements to assess water quality, detect pollution, and manage natural resources effectively. - Renewable Energy and Materials:
As industries focus on sustainable practices, specific gravity plays a role in evaluating new, eco-friendly materials and in optimizing renewable energy systems such as solar panels and wind turbines.
3. Enhanced Research and Educational Tools
- Interactive Learning Platforms:
Online courses, virtual labs, and interactive simulations are making it easier for students and professionals to learn about specific gravity and its applications. - Interdisciplinary Research:
The study of specific gravity spans multiple disciplines—from chemistry and physics to engineering and environmental science—leading to innovative research and cross-disciplinary collaboration.
Conclusion: Embracing the Significance of Specific Gravity
Our comprehensive exploration of what is specific gravity has revealed a concept that is not only fundamental to the sciences but also integral to many aspects of everyday life and industry. Here are the key takeaways:
Definition and Fundamentals:
Specific gravity is a dimensionless ratio that compares the density of a substance to that of a reference material (usually water at 4°C). It is a crucial measure that helps us understand material properties.Historical Evolution:
The concept of specific gravity has evolved from early experiments in density measurement to a sophisticated tool used across various scientific and industrial fields.Diverse Applications:
From quality control in manufacturing and environmental monitoring to brewing and construction, specific gravity is a versatile and indispensable parameter.Modern Relevance:
Advances in digital sensor technology, automation, and sustainable practices are enhancing our ability to measure and utilize specific gravity, driving innovation and efficiency in multiple domains.
Call to Action
Now that you have a comprehensive understanding of what is specific gravity, we encourage you to:
- Reflect: Consider how specific gravity impacts the products you use every day—from the food and beverages you consume to the materials used in construction and technology.
- Explore Further: Dive deeper into topics such as material science, environmental monitoring, and quality control by exploring additional courses, articles, and research on specific gravity.
- Engage: Share your thoughts, experiences, or questions in the comments below. How has understanding specific gravity influenced your perspective on science or everyday life?
- Share: If you found this article informative and engaging, please share it with friends, colleagues, educators, and anyone interested in learning more about the importance of specific gravity.
By mastering the concept of specific gravity, you empower yourself with a deeper understanding of material properties and the scientific principles that shape our world, driving innovation, sustainability, and quality in every aspect of life.
Additional Resources and References
For further exploration of what is specific gravity, here are some reputable sources and additional reading materials:
Books and Academic Texts:
- “Fundamentals of Physics” by David Halliday, Robert Resnick, and Jearl Walker – A comprehensive guide covering the fundamentals of physics, including density and specific gravity.
- “Introduction to Electrodynamics” by David J. Griffiths – Provides an in-depth discussion of material properties and measurement techniques.
- “Materials Science and Engineering: An Introduction” by William D. Callister Jr. and David G. Rethwisch – Explores the properties of materials, including density and specific gravity, with practical examples.
Online Educational Resources:
- Khan Academy – Density and Specific Gravity – Video lessons and exercises on the concepts of density and specific gravity.
- MIT OpenCourseWare – Free course materials on material science and engineering topics, including measurements of density.
- HyperPhysics – An online resource with detailed explanations and diagrams related to specific gravity.
Research Journals and Articles:
- Journal of Applied Physics – Research articles on material properties and experimental measurement techniques.
- Environmental Science & Technology – Studies on water quality and environmental applications involving specific gravity.
- IEEE Xplore Digital Library – Research papers on sensor technology and digital measurement systems.
Workshops and Online Courses:
- Platforms such as Coursera, edX, and Udemy offer courses on materials science, environmental engineering, and quality control that cover the measurement and application of specific gravity.
- Local community colleges and technical institutes often host workshops on material testing and analysis.
Final Thoughts
Specific gravity is a fundamental concept that helps us understand the density and quality of materials, impacting everything from scientific research and industrial processes to everyday products and environmental conservation. By understanding what is specific gravity, you gain insight into the physical properties that drive innovation, sustainability, and quality across diverse fields. Whether you’re measuring the purity of a liquid, designing advanced materials, or ensuring the safety of your surroundings, the principles of specific gravity are indispensable.
Thank you for joining us on this in-depth exploration of specific gravity. We hope this article has deepened your understanding and sparked your curiosity to learn more about the science that shapes our world. If you enjoyed this post, please share it, leave your feedback or questions in the comments below, and help spread the knowledge about the importance of specific gravity.