“What Is PPM” Everything You Need to Know
Have you ever wondered what is ppm and why this seemingly simple unit of measurement is so crucial across science, industry, and everyday life? Imagine trying to measure the quality of your drinking water, the concentration of pollutants in the air, or even the precise amount of a chemical in a reaction without a standardized method for expressing tiny quantities. In this comprehensive guide, we’ll delve into everything you need to know about ppm—its definition, historical background, real-world applications, and modern trends that highlight its importance. Whether you’re a student, a professional in environmental science or engineering, or just a curious mind, understanding what is ppm will empower you to interpret data and make informed decisions.
In this post, you will learn:
- A clear and concise definition of ppm and its essential characteristics.
- The historical and contextual background behind the development of ppm as a unit of measurement.
- An in-depth exploration of ppm’s applications in various fields such as environmental science, chemistry, engineering, and public health.
- Real-world examples, case studies, and scenarios that illustrate what is ppm and how it is used.
- The importance and benefits of understanding ppm for everyday life, scientific research, and industry.
- Answers to common misconceptions and frequently asked questions (FAQs) about ppm.
- Insights into modern relevance and current trends in ppm usage, including advancements in measurement technology and regulatory standards.
By the end of this article, you’ll not only understand what is ppm—parts per million—but also appreciate its role as a critical tool for precise measurement and data analysis in a multitude of contexts.
Introduction: The Significance of PPM in Our World
Picture this: a drop of ink dispersing in a swimming pool, a minuscule amount of a contaminant in your tap water, or the trace gases in our atmosphere. All these examples involve quantities so small that expressing them in everyday terms like percentages or decimals becomes unwieldy. This is where ppm comes in.
PPM, which stands for “parts per million,” is a unit that quantifies extremely small concentrations. It allows scientists, engineers, and regulators to measure trace amounts with high precision. For example, when environmental agencies monitor air quality or water pollution, they often refer to contaminant levels in ppm. Even in everyday products such as cosmetics or food additives, ppm measurements ensure that ingredients remain within safe limits.
But what is ppm exactly? In this post, we’ll break it down into manageable pieces, starting with a straightforward definition and moving through its historical evolution, practical applications, and even the modern technology that makes ppm measurements more accurate than ever. Let’s explore this indispensable metric together!
What Is PPM? A Straightforward Definition
At its most basic level, what is ppm? PPM stands for “parts per million.” It is a unit of measurement used to describe the concentration of one substance in a mixture or solution. Essentially, ppm represents the ratio of one unit of a substance to one million units of the total. Here are the essential characteristics that define ppm:
Ratio-Based Measurement:
PPM is a dimensionless unit because it expresses a ratio. For example, 1 ppm means that there is 1 unit of a substance for every 1,000,000 units of the total.Versatility Across Contexts:
PPM is used in various fields—from measuring pollutants in the air and water to quantifying ingredients in food and chemicals in industrial processes.Expressing Trace Quantities:
PPM is ideal for representing very low concentrations. When the quantities involved are extremely small, using percentages or decimals may be less intuitive.Conversion to Other Units:
Because ppm is a ratio, it can be easily converted to other units. For example, 1 ppm is equivalent to 0.0001% (parts per hundred) or 1 mg/L in water (assuming a density close to that of water).
In summary, when you ask what is ppm, you are referring to a unit that allows us to express minute quantities in a standardized, easy-to-understand format. This simple yet powerful concept is the backbone of precise measurement in many scientific and industrial applications.
Historical and Contextual Background: The Evolution of PPM
Understanding what is ppm also involves looking at its historical development and contextual significance. The need to measure small concentrations with precision has been recognized for centuries, and the concept of parts per million evolved over time as technology and scientific understanding advanced.
Early Beginnings and the Need for Precision
Early Chemistry and Analytical Techniques:
In the early days of chemistry, scientists needed to measure the purity of substances and the presence of impurities. Before the advent of modern analytical instruments, chemists relied on weight ratios and comparative methods to determine concentration. As techniques improved, so did the need for more refined units of measurement.Industrial Revolution and Environmental Awareness:
With the Industrial Revolution came mass production and, unfortunately, increased pollution. The need to monitor pollutants in the air and water became crucial for public health and environmental protection. PPM emerged as a convenient unit to measure the concentration of contaminants such as sulfur dioxide, nitrogen oxides, and other industrial pollutants.
Milestones in the Development of PPM
Standardization in Scientific Research:
By the mid-20th century, as analytical chemistry advanced, the scientific community adopted ppm as a standard unit for expressing concentration. This standardization was crucial for ensuring consistency across research, industry, and regulatory frameworks.Regulatory Implementation:
Government agencies and international organizations began to establish guidelines and limits for various substances in ppm. For example, the U.S. Environmental Protection Agency (EPA) and the World Health Organization (WHO) use ppm to set safe limits for contaminants in air, water, and soil.
Anecdotes and Historical Tidbits
From the Laboratory to the Field:
Early experiments in the laboratory often required precise measurement of trace elements. One famous example involves the detection of lead in drinking water—a task that, even today, relies on ppm measurements to ensure safety standards are met.Technological Advances:
The invention of sensitive instruments such as mass spectrometers and gas chromatographs further refined the ability to measure concentrations in ppm. These tools have transformed environmental monitoring, quality control in manufacturing, and research in fields like pharmacology.
The historical evolution of ppm highlights its role as a vital tool for achieving accuracy and consistency in measurements, a necessity that has only grown as our understanding of environmental and chemical processes has deepened.
In-Depth Exploration: Unpacking “What Is PPM”
Now that we have defined ppm and explored its historical background, let’s dive deeper into its attributes, practical applications, and real-world examples. This section is designed to provide an in-depth look at what is ppm and how it is used across various domains.
1. Understanding PPM as a Unit of Measurement
A. The Mathematics Behind PPM
- Definition in Mathematical Terms:
PPM is defined as the number of parts of a substance per 1,000,000 parts of the whole. Mathematically, if you have a substance A in a mixture M, then: - Examples of Calculation:
- Water Quality: If 2 milligrams of a contaminant are present in 1 liter of water (approximately 1,000,000 milligrams of water), then the concentration is:
- Air Pollutants: In air quality measurements, if there are 3 parts of a pollutant per 1,000,000 parts of air, the concentration is 3 ppm.
B. Converting PPM to Other Units
- Percentage (%):
1 ppm is equivalent to 0.0001% because 1% equals 10,000 ppm. - Milligrams per Liter (mg/L):
In aqueous solutions where the density of water is approximately 1 g/mL, 1 mg/L is roughly equivalent to 1 ppm. - Parts Per Billion (ppb) and Parts Per Trillion (ppt):
These units are used for even lower concentrations. For example, 1 ppm = 1,000 ppb and 1 ppb = 1,000 ppt.
Understanding these conversions is essential for comparing data across different studies and regulatory standards.
2. Applications of PPM Across Various Fields
PPM is used in many disciplines. Let’s explore some of its key applications:
A. Environmental Science
Water Quality Testing:
Regulatory agencies use ppm to measure contaminants in drinking water. For example, the concentration of chlorine, heavy metals (like lead and arsenic), and other pollutants is commonly expressed in ppm. Ensuring that water quality remains within safe ppm limits is critical for public health.Air Pollution Monitoring:
Air quality is often measured in ppm, particularly for gases such as carbon monoxide (CO), sulfur dioxide (SO₂), and nitrogen dioxide (NO₂). Monitoring these levels helps in assessing environmental health and enforcing air quality standards.Soil Contamination:
The concentration of pesticides, heavy metals, or other contaminants in soil is frequently expressed in ppm. This information guides remediation efforts and helps ensure agricultural safety.
B. Chemistry and Industrial Processes
Chemical Reactions:
In chemical manufacturing, controlling the concentration of reactants and catalysts is critical. Expressing these concentrations in ppm allows for precise formulation and quality control.Quality Control:
Industries such as pharmaceuticals, food production, and electronics rely on ppm measurements to maintain product consistency. For example, impurities in a drug must be controlled at the ppm level to meet safety regulations.Material Science:
The composition of alloys, polymers, and other materials is often described in ppm. Trace elements can significantly affect the properties of a material, so precise measurement is essential.
C. Public Health and Safety
Regulatory Standards:
Government agencies establish safe exposure limits for chemicals and pollutants in terms of ppm. For instance, occupational safety guidelines for air contaminants in the workplace are frequently set in ppm to protect workers’ health.Food and Beverage Industry:
PPM is used to ensure that food additives, preservatives, and contaminants remain within safe limits. This ensures consumer safety and helps companies comply with international food safety standards.
D. Everyday Applications
Household Products:
Products such as cleaning agents, cosmetics, and even some medications use ppm to indicate the concentration of active ingredients or potential contaminants.Automotive and Engineering:
In engine oils and coolants, the concentration of additives is critical to performance and longevity. Expressing these concentrations in ppm helps engineers maintain optimal operating conditions.
3. Real-World Examples and Case Studies
Let’s explore some case studies and real-world scenarios to illustrate what is ppm and its practical importance.
Case Study 1: Monitoring Drinking Water Quality
Scenario: A municipal water treatment facility must ensure that the concentration of lead in drinking water does not exceed 15 ppm, as regulated by local environmental standards.
- Methodology:
Water samples are collected at various points in the distribution system. Using advanced spectrometry techniques, the lead concentration is measured and expressed in ppm. - Results:
If a sample shows 12 ppm of lead, it is within the safe limit. However, if a sample shows 18 ppm, immediate corrective actions such as adjusting treatment processes or issuing public warnings are necessary. - Impact:
Continuous monitoring in ppm ensures that water quality remains safe for consumers, reducing the risk of lead poisoning and associated health issues.
Case Study 2: Air Quality and Urban Pollution
Scenario: A city implements an air quality monitoring program to measure the concentration of carbon monoxide (CO) in urban areas. The regulatory limit is set at 9 ppm for an 8-hour exposure period.
- Methodology:
Air sensors are strategically placed across the city to collect data on CO levels throughout the day. These sensors provide real-time measurements in ppm. - Results:
Data indicates that during peak traffic hours, CO levels rise to 7–8 ppm, while during off-peak hours, levels drop to 3–4 ppm. - Impact:
The city uses these measurements to adjust traffic flow, enforce emission controls, and inform residents about air quality, ultimately contributing to better public health outcomes.
Case Study 3: Industrial Quality Control in Pharmaceuticals
Scenario: A pharmaceutical company must ensure that the impurity levels in a new drug formulation are below 5 ppm to meet international safety standards.
- Methodology:
The drug is subjected to rigorous testing using high-performance liquid chromatography (HPLC). The concentration of impurities is measured and reported in ppm. - Results:
Consistent results showing impurity levels below 3 ppm confirm that the manufacturing process is robust and meets safety regulations. - Impact:
Maintaining such low impurity levels in ppm ensures the efficacy and safety of the drug, protecting patients and meeting stringent regulatory requirements.
Importance, Applications, and Benefits of Understanding PPM
Understanding what is ppm is vital not only for scientific accuracy but also for practical decision-making across various domains. Here are some key reasons why grasping the concept of ppm is so important:
1. Enhancing Scientific Precision and Communication
- Standardization of Measurements:
PPM provides a standardized method to express very low concentrations, ensuring consistency in scientific research, industrial processes, and regulatory compliance. - Clarity in Data Interpretation:
When measurements are reported in ppm, it becomes easier to compare data across different studies and industries. This clarity is crucial for effective communication and decision-making.
2. Environmental Protection and Public Health
- Pollution Monitoring:
Accurate measurement of pollutants in air, water, and soil in ppm is essential for protecting ecosystems and human health. It helps regulatory agencies set and enforce safety standards. - Risk Assessment:
By understanding concentrations in ppm, health officials and environmental scientists can assess risks, implement remediation strategies, and design policies that safeguard public well-being.
3. Industrial Efficiency and Quality Control
- Optimizing Production Processes:
In manufacturing, knowing the exact concentration of chemicals or impurities in ppm enables companies to fine-tune their processes, reduce waste, and improve product quality. - Compliance and Safety:
Many industries, from food production to pharmaceuticals, rely on ppm measurements to ensure that products meet strict safety and quality standards, thereby protecting consumers and reducing liability risks.
4. Everyday Applications and Consumer Awareness
- Informed Consumer Choices:
Understanding ppm helps consumers interpret product labels and safety information, whether it’s in the context of air quality monitors, water filters, or cosmetic products. - Enhanced Awareness of Environmental Issues:
As media and governments increasingly report environmental data in ppm, being familiar with this unit allows the public to better understand and react to issues such as pollution alerts or changes in water quality.
Addressing Common Misconceptions and FAQs About PPM
Despite its widespread use, several misconceptions about what is ppm persist. Let’s address some common questions and clear up any confusion.
FAQ 1: What Exactly Does PPM Mean?
- Answer: PPM stands for “parts per million.” It is a unit of measurement that represents the number of parts of a substance found in one million parts of the total. For example, 1 ppm means 1 unit of a substance per 1,000,000 units of the mixture.
FAQ 2: How Is PPM Different from Percentage?
- Answer: While both ppm and percentage are used to describe concentration, they operate on different scales. One percent (%) is equivalent to 10,000 ppm. Thus, ppm is used when the concentration is very small and a percentage would be too large or imprecise.
FAQ 3: Can PPM Be Used for Both Solids and Gases?
- Answer: Yes, ppm is a versatile unit that can be applied to measure the concentration of substances in solids, liquids, and gases. It is commonly used in environmental science to measure contaminants in air and water as well as in industrial quality control.
FAQ 4: Why Is PPM Important in Environmental Monitoring?
- Answer: PPM is critical in environmental monitoring because it allows for the precise measurement of trace contaminants. Even small concentrations (measured in ppm) can have significant impacts on human health and ecosystems, making accurate measurements essential for regulatory compliance and remediation efforts.
FAQ 5: How Do I Convert PPM to Other Units?
- Answer: Conversion from ppm to other units depends on the context:
- To Percent: Divide the ppm value by 10,000 (e.g., 500 ppm = 0.05%).
- To mg/L: In water (with a density of approximately 1 g/mL), 1 ppm is roughly equivalent to 1 mg/L.
- To ppb (Parts per Billion): Multiply the ppm value by 1,000 (e.g., 1 ppm = 1,000 ppb).
Modern Relevance and Current Trends in PPM Measurement
The study and application of what is ppm continue to evolve with advances in technology and increased environmental awareness. Here are some modern trends and developments:
1. Advances in Measurement Technology
High-Precision Instruments:
New analytical instruments such as advanced mass spectrometers, laser-based sensors, and digital gas analyzers are capable of detecting concentrations in ppm with unprecedented accuracy. These innovations are crucial for both laboratory research and field measurements.Real-Time Monitoring:
The development of portable and real-time monitoring devices that measure pollutants in ppm is revolutionizing environmental monitoring. These devices allow for immediate data collection and faster response times in cases of environmental emergencies.
2. Integration in Regulatory Frameworks
Stricter Environmental Standards:
With increasing awareness of climate change and pollution, governments and international bodies are setting stricter limits for contaminants, often expressed in ppm. This drives demand for more accurate measurement and reporting technologies.Global Consistency:
Standardizing the use of ppm across different regions and industries facilitates international cooperation in areas such as environmental protection, public health, and trade regulations.
3. Application in Emerging Fields
Nanotechnology and Material Science:
In fields where even trace impurities can affect material properties, ppm measurements are critical. For instance, the performance of semiconductor devices can hinge on impurity levels measured in parts per million.Biotechnology and Pharmaceuticals:
Advances in biotechnology require precise control of chemical concentrations in cell cultures and drug formulations. PPM remains a fundamental unit in ensuring the safety and efficacy of these products.
4. Public Awareness and Educational Tools
Citizen Science Projects:
Increasingly, citizen science projects involve community members in monitoring local air and water quality. Educational platforms now include modules on ppm, helping the public understand environmental data.Digital Platforms and Apps:
Apps and online tools that convert and explain ppm values make it easier for non-experts to understand scientific measurements and regulatory standards.
Conclusion: Embracing the Precision and Power of PPM
In summary, understanding what is ppm is essential for grasping the details of concentration measurements in science, industry, and everyday life. PPM—parts per million—provides a standardized, precise method for expressing very small quantities, making it indispensable for tasks ranging from monitoring environmental pollutants to ensuring the quality of industrial products.
Key Points Recap
Definition and Function:
PPM stands for parts per million, a unit used to express the ratio of a substance in a mixture, enabling precise measurements even at extremely low concentrations.Historical Evolution:
The concept of ppm has evolved from early analytical methods in chemistry to a standardized unit critical in environmental monitoring, industrial quality control, and public health.Wide-Ranging Applications:
From water and air quality testing to pharmaceutical manufacturing and material science, ppm is used across diverse fields to ensure accuracy and safety.Modern Relevance:
Technological advancements and stricter regulatory standards continue to enhance the application of ppm, making it a vital tool in addressing modern environmental and industrial challenges.Practical Benefits:
Understanding ppm empowers you to interpret scientific data, make informed decisions, and appreciate the precision behind everyday measurements.
Call to Action
Now that you have a comprehensive understanding of what is ppm, why not explore further? Here are a few ways to deepen your knowledge:
- Engage with the Data: Look for real-world examples of ppm measurements in news articles about air quality or water safety. Try comparing local environmental reports to see how ppm values impact your community.
- Share Your Thoughts: Have you encountered ppm in your work or studies? Leave a comment below sharing your experiences or questions about ppm.
- Spread the Word: If you found this post informative, share it with colleagues, friends, or on social media to help others understand the importance of precise measurement in our modern world.
- Further Reading: Subscribe to our newsletter and check out additional resources on environmental science, chemistry, and measurement technology. For more in-depth exploration, consider visiting reputable sites such as the U.S. Environmental Protection Agency (EPA) or the World Health Organization (WHO).
Additional Resources and Further Reading
For those who want to dive deeper into the topic of ppm and its applications, here are some valuable resources:
Books:
- “Environmental Chemistry” by Stanley E. Manahan – Offers an in-depth look at chemical measurements, including ppm, in environmental contexts.
- “Analytical Chemistry: A Modern Approach to Analytical Science” by Robert Kellner et al. – Explores analytical techniques and units of measurement used in laboratories.
Websites:
- EPA Air Quality Monitoring – Provides data and resources on measuring air pollutants in ppm.
- World Health Organization – Water Quality – Offers guidelines and information on acceptable ppm levels for contaminants in water.
- ScienceDirect – Search for academic articles and research papers on ppm in various scientific fields.
Educational Platforms:
- Khan Academy – Features tutorials on chemical measurements and concentration units.
- Coursera – Offers courses in environmental science and analytical chemistry that cover topics including ppm.
Final Thoughts
The unit ppm might seem small, but its impact is enormous. Understanding what is ppm unlocks a level of precision that is fundamental to our understanding of science, environmental stewardship, and quality control in industry. Whether you’re monitoring the purity of your water, ensuring that air quality meets health standards, or controlling the chemical composition of products, ppm is an indispensable tool in our modern world.
By mastering the concept of ppm, you empower yourself with the knowledge to interpret and act upon data that affects everything from public health to global environmental policies. Embrace the power of precision and let your understanding of ppm guide you in making informed, impactful decisions.
Thank you for joining us on this deep dive into what is ppm. We hope this guide has enriched your knowledge and inspired you to further explore the fascinating world of measurement and analysis. Remember, every tiny part matters when it comes to making a big difference.
Share and Engage!
If you found this comprehensive guide on what is ppm helpful, please consider sharing it on social media or with colleagues who might benefit from this information. We invite you to join the conversation by leaving your thoughts, experiences, or questions in the comments below. Your feedback helps us improve and cover more topics that matter to you.
Stay curious, keep learning, and always appreciate the small details—they often make the biggest impact!

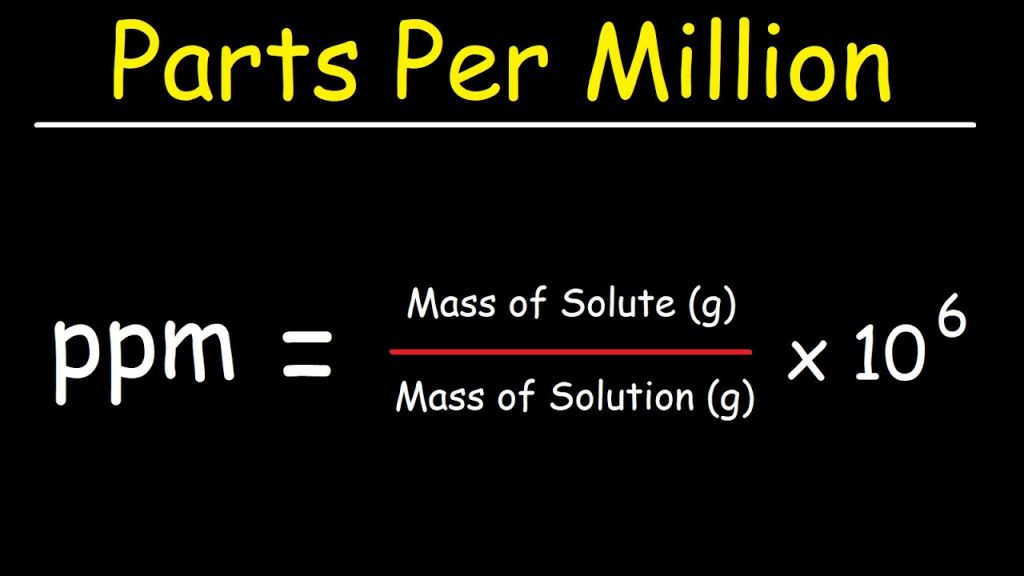

 4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all
4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all