“What is Catenation” – Everything You Need to Know
Have you ever marveled at the seemingly endless chains that make up organic compounds or wondered how atoms come together to form the intricate structures of life? The answer lies in a fascinating chemical phenomenon called catenation. In this comprehensive guide, we’ll explore what is catenation by delving into its definition, historical evolution, scientific principles, and real-world applications. Whether you’re a chemistry student, a science enthusiast, or someone curious about the building blocks of matter, this post will equip you with all the insights you need about catenation.
Introduction: Unlocking the Chains of Nature
Imagine a world where atoms link together to form the long, stable chains that create the very basis of organic chemistry. From the carbon backbones of proteins and DNA to the complex polymers that make up everyday plastics, the phenomenon of catenation is at work behind the scenes. But what is catenation? Why is this ability of atoms to bond with themselves so crucial for life and modern technology? In this post, we will cover:
- A Clear Definition: Learn what catenation is and what characteristics define it.
- Historical Background: Discover the origins of the concept and key milestones in its scientific understanding.
- In-Depth Exploration: Break down the chemical principles behind catenation, including examples of how it shapes the structure of molecules.
- Real-World Applications: See how catenation influences everything from organic chemistry and material science to biotechnology and industrial processes.
- Importance and Benefits: Understand why mastering the concept of catenation is essential for advancements in science and technology.
- Common Misconceptions and FAQs: Get answers to frequently asked questions and clear up myths surrounding catenation.
- Modern Relevance: Explore current research trends and how catenation is evolving in today’s scientific landscape.
By the end of this article, you’ll have a robust understanding of what is catenation and why it’s a cornerstone concept in chemistry and materials science.
What is Catenation? A Straightforward Definition
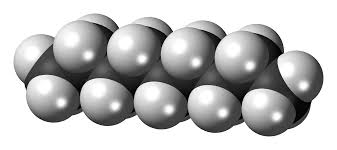
Catenation is the ability of an element to form bonds with itself, creating long chains, rings, or networks of atoms. This phenomenon is most famously associated with carbon, which is renowned for its ability to form complex organic molecules. However, other elements—such as silicon, sulfur, and phosphorus—can also exhibit catenation under certain conditions.
Essential Characteristics of Catenation
- Self-Linking: At its core, catenation is the process by which atoms of the same element bond together. This linking can form linear chains, branched structures, or cyclic rings.
- Bond Strength: The ability to catenate depends on the strength and stability of the bonds between atoms. In carbon, the strong covalent bonds allow for the formation of stable, long chains that are the backbone of organic chemistry.
- Versatility: Catenation is responsible for the vast diversity of molecular structures in organic compounds. It allows for the creation of a myriad of compounds with different properties, which is essential for life and various technological applications.
- Element-Specific: While carbon is the most prominent example, the extent and nature of catenation can vary among elements. The phenomenon is influenced by atomic size, electronegativity, and other factors that affect bond formation.
Understanding what is catenation is fundamental to appreciating how complex molecules are built, paving the way for innovations in everything from medicine to materials engineering.
Historical and Contextual Background
The concept of catenation has been a key part of chemical theory since the early days of modern chemistry. Its exploration has not only deepened our understanding of molecular structures but also driven technological and scientific advancements.
Early Observations and Theoretical Foundations
The Rise of Organic Chemistry:
In the 19th century, chemists began to unravel the mysteries of organic compounds. The discovery that carbon atoms could bond with one another in long chains was pivotal in establishing organic chemistry as a distinct scientific discipline. Early chemists like Friedrich Wöhler and Justus von Liebig made significant contributions by synthesizing organic compounds in the laboratory.Carbon’s Unique Role:
Carbon’s ability to catenate—form chains with itself—was soon recognized as the foundation of organic chemistry. This property allowed for the existence of countless compounds, ranging from simple molecules like methane (CH₄) to complex polymers and biomolecules. The study of these carbon chains led to the development of structural theories that underpin modern organic chemistry.
Milestones in the Evolution of Catenation
Structural Theory Development:
The mid-19th century saw the emergence of structural theory, where chemists like August Kekulé and Archibald Scott Couper proposed that carbon atoms could form long chains and rings. Kekulé’s famous dream of the benzene ring is a legendary anecdote that highlights the imaginative leaps required to understand molecular structures.Advances in Spectroscopy and Crystallography:
The advent of techniques such as X-ray crystallography and spectroscopy in the 20th century allowed scientists to determine the exact structures of complex molecules. These advancements provided concrete evidence of catenation in organic compounds and deepened our understanding of molecular architecture.Polymer Science and Materials Engineering:
In the 20th century, the study of polymers—long chains of repeating molecular units—demonstrated the practical importance of catenation. The development of synthetic polymers revolutionized industries such as plastics, textiles, and electronics, showcasing how catenation can be harnessed for technological innovation.
Notable Historical Anecdotes
Kekulé’s Benzene Ring:
One of the most famous stories in chemistry involves August Kekulé’s vision of the benzene ring. Legend has it that Kekulé dreamed of a snake biting its tail, which inspired his understanding of the cyclic structure of benzene. This revelation was a turning point in organic chemistry, highlighting the power of catenation in creating stable, ring-shaped molecules.The Birth of Polymer Science:
The invention of materials like nylon and polyethylene in the early 20th century was made possible by understanding and manipulating catenation. These breakthroughs transformed manufacturing and led to the development of countless everyday products.
In-Depth Exploration: The Science and Applications of Catenation
To fully appreciate what is catenation, we must delve into its scientific principles and explore how it manifests in various fields.
1. The Chemical Basis of Catenation
Covalent Bonding and Carbon’s Uniqueness
Covalent Bonds:
Catenation primarily involves covalent bonding, where atoms share electrons to achieve stable electron configurations. Carbon’s four valence electrons allow it to form four covalent bonds, creating a versatile and robust framework for building long chains and complex structures.Bond Energy and Stability:
The strength of the covalent bonds between carbon atoms is a key factor in catenation. These bonds are strong enough to maintain the integrity of long chains, yet flexible enough to allow for branching and the formation of rings.
Comparison with Other Elements
Silicon and Catenation:
Silicon, which is in the same group as carbon on the periodic table, can also exhibit catenation. However, silicon-silicon bonds are generally weaker than carbon-carbon bonds, which limits the extent to which silicon can form stable long chains. This difference explains why organic compounds (based on carbon) are far more diverse than their silicon-based counterparts.Other Elements:
Elements like sulfur and phosphorus also show catenation, though typically under specific conditions. For example, sulfur can form cyclic octatomic molecules (S₈), which are commonly found in nature, but these structures are not as versatile as carbon chains.
2. Types of Catenated Structures
Linear and Branched Chains
Linear Chains:
In a linear chain, atoms are bonded in a single, unbranched sequence. This type of structure is common in simple hydrocarbons like alkanes (e.g., methane, ethane, propane).Branched Chains:
In branched chains, side groups are attached to the main chain, creating a more complex structure. Branched molecules can have different physical and chemical properties compared to their linear counterparts, such as lower boiling points and altered reactivity.
Cyclic Structures
Rings and Cycles:
Catenation is not limited to linear arrangements. Atoms can also bond to form closed loops or rings. Benzene, with its six carbon atoms arranged in a ring, is a classic example of a cyclic structure that has significant implications in both chemistry and industry.Fused and Spiro Compounds:
More complex cyclic structures include fused rings (where rings share common atoms) and spiro compounds (where rings are connected through a single atom). These structures are common in many natural and synthetic compounds, including pharmaceuticals.
3. Applications of Catenation in Science and Industry
Organic Chemistry and Pharmaceuticals
Drug Development:
The ability of carbon to form long, stable chains is fundamental to organic chemistry, which in turn is critical for drug design. Many pharmaceutical compounds are built around carbon chains that are modified to interact with specific biological targets.Biomolecules:
Proteins, DNA, and carbohydrates all rely on catenation. The complex structures of these biomolecules—formed by carbon chains—are essential for the functioning of all living organisms.
Material Science and Polymers
Synthetic Polymers:
The development of plastics and other synthetic materials is largely based on the catenation of carbon atoms. Polymers like polyethylene, polypropylene, and polystyrene are created by linking together long chains of carbon-based monomers.High-Performance Materials:
Advances in polymer science have led to the creation of materials with specialized properties, such as high strength, flexibility, and resistance to heat and chemicals. These innovations have applications in aerospace, automotive manufacturing, and everyday consumer products.
Environmental and Technological Applications
Biodegradable Materials:
Research in catenation is driving the development of biodegradable polymers that can help reduce environmental pollution. By designing molecules that break down more easily, scientists aim to create sustainable materials for the future.Nanotechnology:
The principles of catenation are also applied in nanotechnology, where the controlled assembly of molecules is used to create new materials with unique properties at the nanoscale.
Real-World Example:
Consider the role of catenation in the creation of carbon nanotubes. These cylindrical molecules, composed of rolled-up sheets of carbon atoms, exhibit extraordinary strength and electrical conductivity. They are being explored for use in everything from electronics to medical devices.
Importance, Applications, and Benefits of Understanding Catenation
Grasping what is catenation is essential for anyone involved in chemistry, material science, or related fields. Here’s why understanding catenation is so important:
1. Advancing Scientific Research
Fundamental Chemistry:
Catenation is a cornerstone concept in organic chemistry. A deep understanding of how atoms bond with one another is essential for studying chemical reactions, synthesizing new compounds, and advancing our overall knowledge of chemistry.Innovative Materials:
The ability to design and create new materials hinges on understanding catenation. By manipulating the way atoms connect, scientists can develop polymers and other substances with tailored properties for specific applications.
2. Driving Technological Innovation
Pharmaceuticals and Biotechnology:
Many life-saving drugs and medical treatments are based on complex organic molecules whose structures are determined by catenation. Innovations in drug development often rely on the precise control of carbon chains to create molecules with desired biological effects.Nanotechnology and Electronics:
Materials like carbon nanotubes and graphene, which owe their unique properties to the catenation of carbon atoms, are at the forefront of technological advances. These materials are transforming industries from electronics to renewable energy.
3. Practical Applications in Everyday Life
Consumer Products:
The plastics, rubbers, and synthetic fibers that we use daily are all made possible through catenation. Understanding the science behind these materials can lead to improved product designs and better performance.Environmental Impact:
With growing concerns about sustainability, research into biodegradable polymers and recyclable materials relies heavily on catenation. This knowledge helps in developing eco-friendly materials that can reduce pollution and conserve resources.
4. Enhancing Educational and Professional Development
Academic Success:
For students studying chemistry and related fields, mastering the concept of catenation is crucial for academic success. It lays the groundwork for understanding more advanced topics in chemical synthesis and material science.Career Opportunities:
Professionals in fields ranging from pharmaceuticals to materials engineering benefit from a solid understanding of catenation. It is a key concept that underpins many innovative technologies and research projects.
Addressing Common Misconceptions and FAQs About Catenation
Despite its importance, several misconceptions about what is catenation persist. Let’s clarify some common myths and answer frequently asked questions.
Frequently Asked Questions
Q1: Is catenation unique to carbon?
A: While carbon is the most well-known element for its ability to catenate due to its strong covalent bonds and versatile bonding configurations, other elements (such as silicon, sulfur, and phosphorus) can also form chains under certain conditions. However, carbon remains unparalleled in its ability to form diverse and stable long-chain compounds.Q2: Does catenation only occur in organic compounds?
A: Catenation is most commonly associated with organic compounds because of carbon’s central role in organic chemistry. However, the concept can apply to any element capable of bonding with itself, though the extent and stability of such chains vary among different elements.Q3: Why is catenation important for life?
A: The diversity and complexity of life are largely due to carbon’s ability to form long chains and complex molecules. Biomolecules such as proteins, nucleic acids, and carbohydrates are all built on carbon chains formed through catenation, making it essential for the existence of life.Q4: Can catenation occur in non-biological systems?
A: Yes, catenation is a fundamental chemical process that is not limited to biological systems. It plays a crucial role in the creation of synthetic polymers, advanced materials, and nanomaterials, all of which have significant industrial and technological applications.Q5: How is catenation measured or studied in the laboratory?
A: Scientists study catenation through various techniques such as spectroscopy, X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy. These methods allow researchers to analyze the structure and stability of molecular chains, helping to elucidate the mechanisms behind catenation.
Common Misconceptions
Myth: Catenation is only important for chemists and scientists.
Fact: While catenation is a fundamental concept in chemistry, its implications reach far beyond the laboratory. It affects everyday products, technological innovations, and even environmental sustainability.Myth: Only carbon can form long chains.
Fact: Although carbon is the most versatile element for catenation, other elements can also bond with themselves. The extent of catenation, however, is most pronounced in carbon due to its unique bonding properties.Myth: Catenation is a simple concept.
Fact: While the basic idea of atoms bonding with themselves is straightforward, the underlying chemistry is complex and involves intricate interactions that dictate the properties and stability of the resulting molecules.
Modern Relevance and Current Trends in Catenation
As science and technology advance, the study of catenation continues to be a vibrant field of research with significant implications for various industries and scientific disciplines.
Advances in Materials Science and Nanotechnology
Innovative Polymers:
Ongoing research in polymer chemistry focuses on manipulating catenation to create materials with enhanced properties, such as increased strength, flexibility, or thermal stability. These innovations are driving advancements in fields ranging from aerospace to consumer electronics.Nanomaterials:
Carbon-based nanomaterials like carbon nanotubes and graphene are prime examples of the power of catenation. Their unique properties, derived from the way carbon atoms are arranged, are revolutionizing electronics, energy storage, and even biomedical applications.
Environmental and Sustainable Technologies
Biodegradable Polymers:
With a growing emphasis on sustainability, researchers are exploring ways to design polymers that degrade naturally without harming the environment. Understanding catenation is key to developing these eco-friendly materials.Recycling and Circular Economy:
Advances in understanding molecular structures through catenation contribute to better recycling technologies and the development of materials that can be reused, helping to minimize waste and environmental impact.
Educational and Research Innovations
Interactive Learning Tools:
Digital platforms and virtual laboratories are making it easier for students to visualize and experiment with catenation. Interactive simulations help demystify the process of molecular chain formation and enhance overall comprehension.Interdisciplinary Research:
The study of catenation is increasingly interdisciplinary, combining insights from chemistry, physics, biology, and engineering. This collaborative approach is leading to breakthroughs in material science, medicine, and environmental technology.
Global and Industrial Impact
High-Tech Manufacturing:
Industries are leveraging the principles of catenation to develop new materials and products that improve performance and durability. This includes everything from high-performance plastics to advanced composites used in construction and transportation.Pharmaceutical Innovations:
In drug development, understanding catenation helps chemists design complex molecules that can target specific biological processes, leading to more effective and targeted therapies.
Conclusion: Embracing the Chains of Innovation
In our exploration of what is catenation, we have unraveled the fascinating process by which atoms, particularly carbon, bond together to form complex structures. This fundamental chemical phenomenon is at the heart of organic chemistry, enabling the diversity of life and driving countless innovations in technology and industry.
Key Takeaways
Definition and Core Concepts:
Catenation is the ability of atoms to bond with themselves, forming long chains, rings, or networks. This process is critical in organic chemistry and is most notably exhibited by carbon.Historical Evolution:
From the early days of organic chemistry to modern advancements in polymer science and nanotechnology, catenation has played a pivotal role in shaping our understanding of molecular structures.Wide-Ranging Applications:
The implications of catenation extend from the natural world—where it underpins the structure of biomolecules—to the development of synthetic materials and cutting-edge technologies in various industries.Modern Relevance:
Current research and technological trends continue to harness the power of catenation, driving innovations in sustainable materials, nanotechnology, and high-performance polymers that are transforming our everyday lives.
Call-to-Action
Now that you have a comprehensive understanding of what is catenation, we encourage you to explore further. Whether you’re a student diving deeper into organic chemistry, a professional in materials science, or simply curious about how the molecules around you come together, keep asking questions and seeking new knowledge. Share your thoughts, insights, or any questions in the comments below. If you found this post informative, please share it with friends, colleagues, and fellow science enthusiasts, and subscribe for more in-depth explorations of the fascinating concepts that shape our world.
Additional Resources and Further Reading
For those interested in delving deeper into what is catenation, here are some reputable resources and further reading suggestions:
- Books:
- Organic Chemistry by Paula Yurkanis Bruice – Provides a comprehensive overview of the principles of organic chemistry, including the concept of catenation.
- Introduction to Polymer Chemistry by Charles E. Carraher – Explores the role of catenation in the formation of polymers and synthetic materials.
- Carbon: The Backbone of Life by Colin Tudge – An engaging exploration of carbon’s unique properties and its ability to catenate, forming the basis of organic compounds.
- Online Resources:
- Khan Academy – Organic Chemistry – Offers lessons and videos that explain the basics of organic molecules and catenation.
- ChemLibreTexts – A comprehensive resource for learning about organic chemistry and the concept of catenation.
- Royal Society of Chemistry – Provides articles, research papers, and news related to advancements in chemical research, including studies on catenation.
- Educational Platforms:
- Coursera and edX offer courses in organic chemistry and materials science that cover the principles of catenation and its applications.
- TED Talks and YouTube channels such as “Periodic Videos” provide engaging visual content on chemical bonding and molecular structures.
Final Thoughts
Catenation is the unseen force that links atoms together, forming the intricate molecular chains that give rise to the diversity of organic life and the innovative materials that drive modern technology. By understanding what is catenation, you not only gain insight into a key scientific concept but also appreciate the elegant complexity of the natural world and the transformative potential of chemistry. Embrace the study of catenation, and let it inspire you to explore the endless possibilities of molecular creativity.
Thank you for joining us on this in-depth exploration of catenation. Stay curious, keep learning, and continue to celebrate the amazing chains of innovation that shape our world. If you enjoyed this post, please share it, subscribe for more insightful content, and leave your thoughts and questions in the comments below!