What Is a Catalyst? Everything You Need to Know
Have you ever wondered, what is a catalyst? This question might seem simple at first, but it opens the door to a concept that is vital in chemistry, biology, industry, and even everyday life. Catalysts are the silent engines that accelerate reactions and processes without being consumed themselves. In this comprehensive guide, we’ll explore everything you need to know about catalysts. We’ll define the term, delve into its historical evolution, and examine its diverse applications and benefits. Whether you’re a student, a professional, or simply a curious mind, understanding what is a catalyst is essential for appreciating how these remarkable agents drive progress and innovation across various fields.
Introduction: The Dynamic World of Catalysts
Imagine a busy kitchen where a master chef uses a special spice blend to transform a simple dish into an extraordinary meal. Similarly, in the realm of science and industry, catalysts play a transformative role—they accelerate reactions, improve efficiencies, and even open the door to entirely new processes. But what is a catalyst exactly? Is it simply a substance that speeds up a chemical reaction, or does it have broader implications in our lives and the natural world?
A Captivating Fact to Spark Your Curiosity
Did you know that the catalytic converter in your car reduces harmful emissions by up to 90% by facilitating chemical reactions that convert toxic gases into less harmful substances? This everyday application of catalysts not only helps protect the environment but also illustrates the profound impact catalysts have on technology and sustainability.
What This Article Will Cover
In this extensive post, we will explore:
- A Clear Definition: A straightforward explanation of what a catalyst is, including its essential properties.
- Historical and Contextual Background: The evolution of catalysts from early scientific discoveries to modern innovations.
- In-Depth Exploration: Detailed analysis of various types of catalysts, such as heterogeneous, homogeneous, and biological catalysts (enzymes), complete with real-world examples and case studies.
- Importance, Applications, and Benefits: An examination of why catalysts are crucial in fields like chemistry, industry, environmental science, and everyday life.
- Common Misconceptions and FAQs: Clarifications on frequently misunderstood aspects of catalysts.
- Modern Relevance and Current Trends: A look at recent advancements and ongoing research in catalysis, including green catalysis and nanocatalysis.
- Conclusion and Call-to-Action: A succinct summary of key points and an invitation to further explore and discuss the topic.
By the end of this guide, you will have a robust and nuanced understanding of what is a catalyst and why these substances are fundamental to both scientific progress and practical applications in our modern world.
What Is a Catalyst? A Straightforward Definition
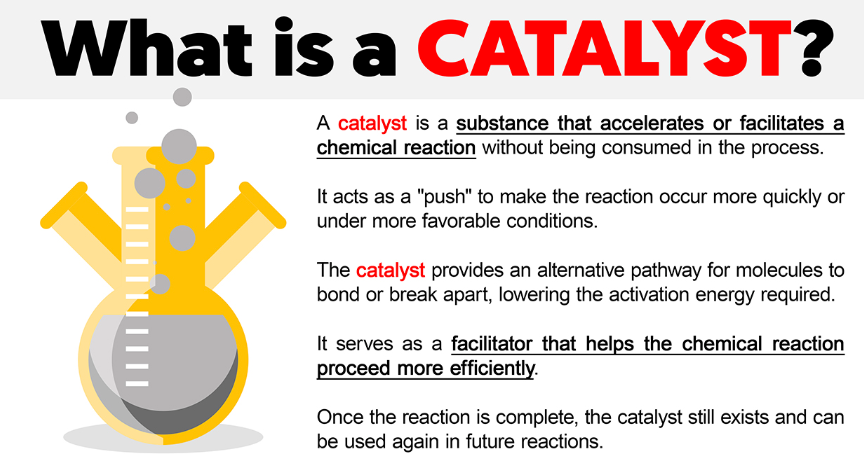
At its most basic level, a catalyst is a substance that increases the rate of a chemical reaction without being permanently altered or consumed in the process. In simpler terms, catalysts make reactions happen faster or more efficiently, often by providing an alternative reaction pathway with a lower activation energy.
Essential Characteristics of a Catalyst
Acceleration of Reactions:
Catalysts speed up chemical reactions by lowering the activation energy required, enabling reactions to occur at lower temperatures or with less energy input.Not Consumed in the Reaction:
Unlike reactants, a catalyst remains unchanged at the end of the reaction. It can be used repeatedly, which makes it an efficient agent in many industrial and biological processes.Selectivity:
Many catalysts are highly selective, meaning they facilitate specific reactions over others. This selectivity is crucial in processes where precision is key, such as in pharmaceutical synthesis.Reusability:
Because catalysts are not consumed during reactions, they can be recovered and reused, contributing to economic efficiency and sustainability in industrial processes.
Thus, what is a catalyst? It is a powerful and versatile tool that facilitates chemical and biological transformations, making processes faster, more efficient, and often more environmentally friendly.
Historical and Contextual Background: The Evolution of Catalysts
Understanding what is a catalyst requires a journey through history. The concept of catalysis has evolved over centuries, from early empirical observations to the sophisticated theories and technologies of today.
Early Discoveries and Theoretical Foundations
Ancient Observations
Early Uses in Alchemy:
Long before modern chemistry, alchemists observed that certain substances could speed up reactions. Although their explanations were not scientifically rigorous, these early observations laid the groundwork for the concept of catalysis.Natural Catalysts:
Nature itself provided early examples of catalysis. For instance, certain minerals and metals were found to accelerate reactions, a phenomenon later understood as catalytic activity.
The Birth of Catalysis as a Scientific Field
Jöns Jacob Berzelius and the Term “Catalysis”:
The term “catalysis” was coined in the early 19th century by the Swedish chemist Jöns Jacob Berzelius. Berzelius’s work helped establish the idea that catalysts accelerate chemical reactions without being consumed.The Work of Wilhelm Ostwald:
In the late 19th and early 20th centuries, Nobel laureate Wilhelm Ostwald significantly advanced the study of catalysis. His research laid the foundation for understanding the kinetics of catalytic reactions and the factors affecting catalytic efficiency.
Milestones in Modern Catalysis
Development of Heterogeneous and Homogeneous Catalysis:
The differentiation between heterogeneous catalysts (which exist in a different phase than the reactants) and homogeneous catalysts (which exist in the same phase) was a major breakthrough. This classification has allowed for more targeted applications and improvements in catalytic processes.Biocatalysis and Enzymes:
The discovery and study of enzymes, which are natural catalysts in biological systems, revolutionized both biology and industrial processes. Enzymes have become essential in fields such as biotechnology, medicine, and food production.
Notable Historical Anecdotes
The Haber-Bosch Process:
One of the most important industrial applications of catalysis is the Haber-Bosch process for synthesizing ammonia from nitrogen and hydrogen. This process, which relies on an iron-based catalyst, is critical for fertilizer production and has had a profound impact on global agriculture and food security.Catalytic Converters:
The development of catalytic converters for automobiles in the 1970s is another landmark. These devices use catalysts to convert harmful pollutants from exhaust gases into less harmful substances, significantly reducing air pollution and improving public health.
These historical milestones illustrate the evolving understanding of what is a catalyst and highlight the transformative impact that catalysts have had on science, industry, and society.
In-Depth Exploration: Types, Attributes, and Categories of Catalysts
Catalysts are not a one-size-fits-all concept—they come in many forms and serve a wide variety of functions. In this section, we will break down the key types and attributes of catalysts, providing real-world examples and case studies to illustrate their applications.
1. Types of Catalysts in Chemistry
A. Heterogeneous Catalysts
Definition:
Heterogeneous catalysts exist in a different phase than the reactants. Typically, these are solid catalysts that accelerate reactions in gaseous or liquid phases.Mechanism of Action:
The reaction occurs on the surface of the catalyst. The reactants adsorb onto the surface, react, and then desorb as products.Examples:
- The Haber-Bosch Process:
Uses an iron-based catalyst to synthesize ammonia. - Catalytic Converters:
Use platinum, palladium, and rhodium to convert toxic gases in automobile exhaust.
- The Haber-Bosch Process:
Advantages:
- Easy separation of catalyst and products.
- Generally more stable and easier to handle.
Limitations:
- Surface deactivation over time.
- Often less selective than homogeneous catalysts.
B. Homogeneous Catalysts
Definition:
Homogeneous catalysts exist in the same phase as the reactants, usually in solution. This allows for uniform mixing and often higher selectivity in reactions.Mechanism of Action:
The catalyst interacts directly with the reactants at the molecular level in the same phase, often forming intermediate compounds.Examples:
- Acid Catalysis:
Sulfuric acid is commonly used as a catalyst in esterification reactions. - Organometallic Catalysts:
Used in processes such as hydroformylation and polymerization.
- Acid Catalysis:
Advantages:
- High selectivity and efficiency.
- Easier to study mechanistically due to uniform conditions.
Limitations:
- Separation of the catalyst from the product can be challenging.
- May require careful control of reaction conditions.
C. Biocatalysts (Enzymes)
Definition:
Enzymes are biological catalysts that speed up biochemical reactions. They are highly specific and work under mild conditions.Mechanism of Action:
Enzymes lower the activation energy of reactions by stabilizing transition states, often through precise molecular interactions.Examples:
- Digestive Enzymes:
Such as amylase and protease, which break down food components. - DNA Polymerase:
Critical for DNA replication in living organisms.
- Digestive Enzymes:
Advantages:
- High specificity and efficiency.
- Operate under environmentally friendly conditions (ambient temperature, neutral pH).
Limitations:
- Sensitive to environmental changes (temperature, pH).
- Can be expensive to produce in large quantities.
2. Catalysts in Industrial and Environmental Applications
A. Industrial Catalysis
Role in Manufacturing:
Catalysts are at the heart of many industrial processes. They enable the efficient synthesis of chemicals, fuels, and materials, significantly reducing energy consumption and production costs.Key Applications:
- Petrochemical Refining:
Catalytic cracking converts heavy hydrocarbons into lighter, more valuable products like gasoline. - Polymerization:
Catalysts drive the formation of polymers such as plastics and synthetic rubbers. - Pharmaceutical Synthesis:
Catalytic reactions are used to produce active pharmaceutical ingredients (APIs) with high purity and selectivity.
- Petrochemical Refining:
Case Study: Catalytic Converters in Automobiles
Catalytic converters use a combination of platinum-group metals to convert harmful emissions—such as carbon monoxide and nitrogen oxides—into less toxic substances. This innovation has played a pivotal role in reducing air pollution and has become a standard component in modern vehicles.
B. Environmental Catalysis
Pollution Control:
Catalysts are used in various environmental applications to reduce emissions and mitigate pollution. They facilitate reactions that break down pollutants into harmless substances.Key Applications:
- Water Treatment:
Catalysts are employed in processes like advanced oxidation to remove contaminants from water. - Air Purification:
Catalytic filters help remove volatile organic compounds (VOCs) and other pollutants from industrial emissions. - Green Chemistry:
The use of catalysts in chemical reactions minimizes waste and reduces the need for harsh reaction conditions, contributing to more sustainable manufacturing practices.
- Water Treatment:
Real-World Example: Industrial Waste Treatment
Many industrial plants now use catalytic processes to treat waste gases and liquids, reducing environmental impact and complying with stringent regulatory standards.
3. Catalysts in Everyday Life
A. Catalysts in Consumer Products
Household Cleaners:
Enzymatic cleaners use biocatalysts to break down stains and organic matter, offering an eco-friendly alternative to harsh chemicals.Food Processing:
Enzymes are widely used in the food industry to improve texture, flavor, and shelf life. For instance, amylase is used in baking to break down starches into sugars.
B. Catalysts in Medicine and Healthcare
Diagnostic Tools:
Enzymatic reactions are fundamental to many diagnostic tests, such as blood glucose monitoring.Therapeutic Applications:
Enzyme replacement therapies help treat conditions where a patient is deficient in a specific enzyme, improving health outcomes and quality of life.
C. Catalysts in Renewable Energy
Fuel Cells:
Catalysts are essential components in fuel cells, where they facilitate the reactions that convert hydrogen and oxygen into water, generating electricity with minimal emissions.Solar Energy Conversion:
In the field of photovoltaics, catalysts are used to improve the efficiency of converting sunlight into electrical energy.
Importance, Applications, and Benefits of Understanding What Is a Catalyst
Understanding what is a catalyst is crucial because catalysts are the driving force behind many processes that impact our lives on multiple levels. Here are some key areas where catalysts are significant:
1. Accelerating Chemical Reactions
Efficiency Gains:
Catalysts lower the activation energy required for reactions, enabling processes to occur more quickly and with lower energy inputs. This efficiency is vital for industrial manufacturing, where even small improvements can lead to substantial cost savings.Environmental Benefits:
By enabling reactions to proceed under milder conditions, catalysts reduce the need for extreme temperatures and pressures, lowering energy consumption and minimizing greenhouse gas emissions.
2. Driving Industrial Innovation
Chemical Production:
Catalysts are indispensable in the production of chemicals, fuels, polymers, and pharmaceuticals. Innovations in catalysis have led to more sustainable production methods and the development of new materials and products.Economic Impact:
The use of catalysts in industrial processes drives economic growth by improving productivity, reducing waste, and creating new market opportunities. The Haber-Bosch process, for example, has been pivotal in supporting global agriculture by providing a cost-effective method for producing ammonia-based fertilizers.
3. Enhancing Environmental Protection
Pollution Reduction:
Catalysts play a key role in reducing industrial emissions and treating waste. From catalytic converters in vehicles to catalytic reactors in power plants, these devices help transform harmful pollutants into benign substances.Sustainable Practices:
The development of green catalysis techniques is a cornerstone of sustainable chemistry, enabling cleaner production methods and reducing the environmental footprint of chemical processes.
4. Advancing Healthcare and Biotechnology
Diagnostic and Therapeutic Applications:
Enzymes, as natural catalysts, are central to many diagnostic tests and therapeutic treatments. They not only facilitate essential biological reactions but also offer targeted approaches in treating diseases.Biotechnology and Research:
Catalysts are essential tools in biotechnological research, driving innovations that lead to improved agricultural practices, environmental remediation, and medical breakthroughs.
5. Fostering Innovation and Knowledge
Interdisciplinary Collaboration:
The study and application of catalysts foster collaboration between chemists, biologists, engineers, and environmental scientists. This interdisciplinary approach accelerates innovation and leads to the development of cutting-edge technologies.Educational Importance:
A solid understanding of catalysis is fundamental for students and professionals in STEM fields. It provides critical insights into reaction mechanisms, energy transfer, and the principles of chemical kinetics.
Addressing Common Misconceptions and FAQs About Catalysts
Despite their widespread use, several misconceptions surround what is a catalyst. Here are some common questions and clarifications:
FAQ 1: Are Catalysts Consumed in Reactions?
Answer:
- Misconception:
Some people mistakenly believe that catalysts are used up during a reaction. - Clarification:
In reality, catalysts are not consumed in the reaction. They facilitate the reaction and then return to their original state, allowing them to be reused repeatedly.
FAQ 2: Do Catalysts Change the Equilibrium of a Reaction?
Answer:
- Misconception:
It is often assumed that catalysts shift the equilibrium position of a reaction. - Clarification:
Catalysts do not change the equilibrium; they only help the reaction reach equilibrium faster by lowering the activation energy. The equilibrium concentrations of reactants and products remain unchanged.
FAQ 3: Can Any Substance Act as a Catalyst?
Answer:
- Misconception:
There is sometimes confusion about what substances can be catalysts. - Clarification:
Only substances that can lower the activation energy without being permanently altered or consumed can act as catalysts. This property is intrinsic to catalysts, whether they are metals, enzymes, or complex organometallic compounds.
FAQ 4: Are Catalysts Expensive to Use?
Answer:
- Misconception:
Due to their sophisticated nature, some assume catalysts are prohibitively expensive. - Clarification:
While some catalysts, particularly those used in industrial processes, may require significant investment, their reusability and the efficiency gains they provide often result in overall cost savings.
Quick Misconceptions at a Glance
- Myth: Catalysts are a magic bullet that can make any reaction fast.
Reality: Catalysts speed up reactions by lowering activation energy but cannot force reactions that are thermodynamically unfavorable. - Myth: Catalysis only matters in laboratories and industry.
Reality: Catalysts are integral to everyday life, from reducing car emissions to aiding digestion through enzymes. - Myth: All catalysts are the same.
Reality: There are many types of catalysts—heterogeneous, homogeneous, and biological—each with unique properties and applications.
Modern Relevance and Current Trends in Catalysis
In today’s rapidly evolving world, the study and application of catalysts are at the forefront of many scientific and industrial breakthroughs. Let’s explore some modern trends and how they are shaping the future of catalysis.
1. Green Catalysis and Sustainability
Eco-Friendly Processes:
The development of green catalysis aims to reduce the environmental impact of chemical processes. By using catalysts that work under milder conditions and generate less waste, industries can produce chemicals and materials in a more sustainable manner.Renewable Resources:
Research is focusing on using catalysts to convert renewable feedstocks into valuable chemicals. For example, catalysts are being developed to transform biomass into biofuels and bioplastics, reducing reliance on fossil fuels.Case Study:
Catalytic converters in automobiles not only reduce emissions but also play a role in the development of cleaner transportation technologies. Innovations in catalyst design continue to improve efficiency and lower environmental impact.
2. Advances in Nanocatalysis
Nanotechnology Integration:
Nanocatalysts, which operate at the nanoscale, offer enhanced surface area and reactivity. These catalysts have the potential to revolutionize industries by providing unprecedented levels of efficiency and selectivity.Applications:
Nanocatalysts are being used in energy conversion, environmental remediation, and pharmaceutical synthesis. Their unique properties allow for faster reactions and lower energy consumption.Future Trends:
As nanotechnology advances, we can expect even more sophisticated catalysts that are tailored for specific reactions, further pushing the boundaries of what is achievable in catalysis.
3. Biocatalysis and Enzyme Engineering
Customized Enzymes:
Advances in biotechnology and genetic engineering have enabled scientists to design and optimize enzymes for specific applications. These biocatalysts are crucial in fields such as pharmaceuticals, food production, and waste management.Medical and Industrial Applications:
Enzyme-based catalysts are used to manufacture complex drugs, enhance food processing techniques, and develop environmentally friendly cleaning products.Impact:
Biocatalysis not only offers efficiency and specificity but also operates under conditions that are safer and more sustainable compared to traditional chemical processes.
4. Digital and Computational Catalysis
Modeling and Simulation:
Modern computational techniques allow scientists to model catalytic reactions at the molecular level. This approach helps in designing new catalysts and understanding reaction mechanisms in detail.Artificial Intelligence (AI):
AI and machine learning algorithms are increasingly being used to predict catalyst performance and optimize reaction conditions, accelerating the discovery of new catalytic materials.Integration with Industry:
These digital tools are transforming how research and development in catalysis are conducted, leading to faster innovation cycles and more efficient industrial processes.
5. Global Impact and Economic Trends
Market Growth:
The global market for catalysts is expanding rapidly due to increasing industrialization, the push for sustainable manufacturing, and advancements in technology. This growth is evident in sectors such as automotive, chemical manufacturing, and energy production.International Collaborations:
Global initiatives and collaborations are driving research in catalysis, as nations and industries work together to develop solutions for common environmental and economic challenges.Policy and Regulation:
Government policies aimed at reducing emissions and promoting green technologies are fostering increased investment in catalytic research and development.
Conclusion: Embracing the Transformative Power of Catalysts
Our exploration of what is a catalyst has taken us on a fascinating journey from the fundamental principles of chemical kinetics to the cutting-edge innovations driving sustainable industry and healthcare. Catalysts are far more than mere substances that speed up reactions—they are the linchpins of modern science and technology, enabling processes that improve efficiency, reduce environmental impact, and drive economic growth.
Key Takeaways
- Comprehensive Definition:
A catalyst is a substance that accelerates the rate of a chemical reaction without being consumed. It works by lowering the activation energy and can be reused repeatedly, making it a vital component in many processes. - Historical Evolution:
From early discoveries by alchemists and pioneering work by scientists like Berzelius and Ostwald to modern applications in industrial chemistry and biotechnology, the concept of catalysis has evolved dramatically. - Diverse Applications:
Catalysts are used in everything from automotive catalytic converters and industrial chemical synthesis to biological processes and renewable energy. Their versatility makes them indispensable in a wide range of fields. - Modern Relevance:
With advancements in nanotechnology, biocatalysis, and computational modeling, the future of catalysis is bright. Innovations in green catalysis and digital techniques are paving the way for more sustainable and efficient processes. - Benefits and Impacts:
Understanding what is a catalyst not only enriches our knowledge of chemistry and biology but also has practical implications for industry, the environment, and our daily lives.
Call to Action
Now that you have a comprehensive understanding of what is a catalyst, consider how this knowledge might benefit you:
- For Students and Educators:
Dive deeper into catalysis through laboratory experiments, advanced courses, and research projects to explore its principles and applications. - For Industry Professionals:
Stay abreast of the latest developments in catalytic technology to drive innovation, improve efficiency, and reduce environmental impacts in your operations. - For Informed Citizens:
Recognize the role of catalysts in everyday products—from cleaner cars to safer medicines—and support initiatives that promote sustainable and innovative technologies. - For Researchers and Innovators:
Embrace interdisciplinary collaboration and use digital tools to push the boundaries of catalytic science, contributing to breakthroughs in energy, healthcare, and environmental protection.
If you found this guide on what is a catalyst insightful, please share it with colleagues, friends, and anyone interested in the transformative power of catalysts. Your feedback, comments, and questions are invaluable in furthering the conversation about this critical topic.
Additional Resources and Further Reading
For those eager to explore more about catalysts, here are some reputable resources and recommended readings:
Books
- “Catalysis” by B. Viswanathan and M. V. R. Rao
A comprehensive introduction to the principles and applications of catalysis. - “The Catalyst Conversation” by various authors
An insightful collection of essays on the role of catalysts in modern industry and technology. - “Principles and Practice of Heterogeneous Catalysis” by J. M. Thomas and W. J. Thomas
A detailed resource on the practical aspects and industrial applications of heterogeneous catalysis.
Online Articles and Journals
- Wikipedia – Catalyst:
Wikipedia: Catalyst offers a thorough overview of catalysts, their history, and applications. - ScienceDirect and Wiley Online Library:
Search for scholarly articles on catalysis to explore recent research and reviews. - The Journal of Catalysis:
A peer-reviewed journal featuring the latest developments in the field of catalysis.
Websites and Online Courses
- Coursera and edX:
These platforms offer courses in catalysis, green chemistry, and industrial chemistry that cover the fundamentals and advanced topics. - Khan Academy – Chemistry:
Provides free lessons on chemical reactions and kinetics, which lay the groundwork for understanding catalysis. - TED Talks on Chemistry and Innovation:
Explore talks that discuss the impact of catalysts on technology and sustainability.
Final Thoughts
The exploration of what is a catalyst has revealed a concept that is as fascinating as it is essential. Catalysts drive the chemical reactions that power everything from everyday products to advanced industrial processes. They are the unsung heroes in automotive systems, environmental protection, healthcare, and renewable energy. By accelerating reactions without being consumed, catalysts exemplify efficiency and sustainability.
As you continue your journey into the world of science and technology, remember that understanding catalysts can open doors to innovations that have the potential to transform our future. Whether you are a student, a professional, or an informed citizen, the principles of catalysis offer insights into how seemingly small changes can have a profound impact on the world.


 4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all
4.1 Attribution Theory and Person Perception: Why We Judge People the Way We Do (Even When We’re Totally Wrong) Let’s be honest. We’ve all



