Unit FRQ: Cellular Energetics and Glycolysis
Overview of Cellular Metabolism FRQ
In this free-response question, we explore different aspects of cellular energetics, focusing on glycolysis, the Krebs cycle, and the efficiency of ATP production. These questions help students think deeply about the biochemical pathways that support life and understand the evolution of metabolic processes.
Free Response Question
Setup
Cellular respiration involves several steps including glycolysis, the Krebs cycle, and the electron transport chain (ETC). Glycolysis takes place in the cytosol and produces ATP and NADH, which are used later to generate additional ATP. The Krebs cycle occurs in the mitochondria of eukaryotes and generates electron carriers (NADH and FADH2) that fuel the ETC, ultimately resulting in the formation of ATP.
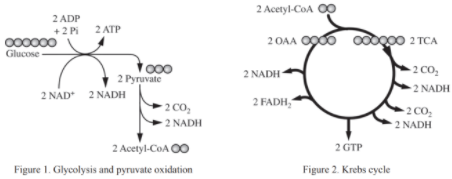
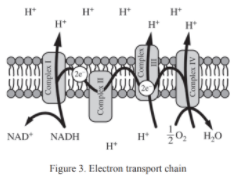
The following diagrams illustrate the steps of glycolysis and pyruvate oxidation (Figure 1), the Krebs cycle (Figure 2), and the electron transport chain (Figure 3). Consider the metabolic processes depicted as you answer the questions below.
Questions
(a) Justify why glycolysis is believed to be an ancient metabolic pathway that originated in the common ancestor of all living organisms. Use the following observations to support your answer.
- Nearly all existing organisms perform glycolysis.
- Glycolysis occurs under anaerobic conditions.
- Glycolysis occurs only in the cytosol.
Answer (a):
Glycolysis is considered an ancient metabolic pathway for several reasons. First, the fact that nearly all existing organisms perform glycolysis suggests that this process originated early in the evolution of life and was passed down from a common ancestor. Since glycolysis occurs under anaerobic conditions, it likely evolved when the Earth’s atmosphere had little or no oxygen. The fact that glycolysis takes place in the cytosol, rather than in specialized organelles, further supports its ancient origin, as the earliest organisms were simple and did not have membrane-bound organelles.
(b) A researcher estimates that, in a certain organism, the complete metabolism of glucose produces 30 molecules of ATP per molecule of glucose. The energy released from the oxidation of glucose under standard conditions is 686 kcal/mol, and the energy released from ATP hydrolysis is 7.3 kcal/mol.
- Calculate the total energy available from the hydrolysis of 30 moles of ATP.
- Calculate the efficiency of ATP production from one mole of glucose in this organism.
- Describe what happens to the excess energy released during glucose metabolism.
Answer (b):
Total Energy Calculation:
The total energy available from the hydrolysis of 30 moles of ATP can be calculated as:
30moles×7.3kcal/mol=219kcalEfficiency Calculation:
The efficiency of ATP production can be calculated using the ratio of energy captured in ATP to the total energy available from glucose:
Efficiency =Excess Energy:
The excess energy not stored in ATP is released as heat, which contributes to maintaining body temperature in organisms. This heat is particularly important in endothermic organisms, such as mammals, which rely on metabolic heat to maintain a stable internal temperature.
(c) The enzymes of the Krebs cycle function in the cytosol of bacteria, but among eukaryotes, the enzymes function mostly in the mitochondria. Pose a scientific question that connects the subcellular location of the Krebs cycle enzymes to the evolution of eukaryotes.
Answer (c):
“How did the relocation of Krebs cycle enzymes from the cytosol in prokaryotes to the mitochondria in eukaryotes contribute to the increased efficiency of ATP production and cellular complexity in eukaryotic organisms?”
This question connects the evolutionary advantage of compartmentalization to the development of more complex cellular structures in eukaryotes, suggesting that mitochondria provide an isolated environment that optimizes the Krebs cycle and enhances ATP production.
Figures Reference
- Figure 1: Glycolysis and Pyruvate Oxidation
Shows the breakdown of glucose into pyruvate, generating ATP and NADH. - Figure 2: Krebs Cycle
Illustrates how acetyl-CoA is further oxidized, producing NADH, FADH2, and GTP. - Figure 3: Electron Transport Chain (ETC)
NADH and FADH2 donate electrons, which travel through the ETC, creating a proton gradient used by ATP synthase to produce ATP.
Key Concepts Recap
- Glycolysis is a highly conserved metabolic pathway, indicating its origin in early evolutionary history.
- ATP production efficiency depends on the balance between energy capture and energy lost as heat.
- The evolution of mitochondria and the compartmentalization of metabolic pathways played a key role in the efficiency and complexity of eukaryotic cells.
Want More Practice?
Check out other FRQs and resources to strengthen your understanding of cellular energetics and evolution. Practicing with diagrams like Figures 1-3 can also help in mastering complex processes like glycolysis, the Krebs cycle, and oxidative phosphorylation.
Electron Transport Chain Overview: The electron transport chain (ETC) is a series of protein complexes and other molecules embedded in the inner mitochondrial membrane that are crucial for ATP production. It is the final stage of cellular respiration where high-energy electrons from NADH and FADH2 are transferred through multiple complexes, ultimately resulting in the production of ATP and water.
Improvised Diagram Elements and Descriptions:
Complex I: NADH Dehydrogenase
- Role: Receives high-energy electrons from NADH, resulting in the oxidation of NADH to NAD⁺.
- Electron Transfer: Electrons are transferred from NADH to Complex I.
- Proton Pumping: Complex I uses the energy of the electrons to pump protons (H⁺) from the mitochondrial matrix to the intermembrane space, creating a proton gradient.
Ubiquinone (Q)
- Role: A mobile electron carrier that transfers electrons from Complex I to Complex III.
- Description: Ubiquinone is a small lipid-soluble molecule, allowing it to move within the membrane.
Complex II: Succinate Dehydrogenase
- Role: Accepts electrons from FADH₂, which is produced in the Krebs cycle.
- Note: Unlike Complex I, Complex II does not pump protons into the intermembrane space.
- Electron Transfer: Electrons are transferred from Complex II to ubiquinone (Q).
Complex III: Cytochrome bc1 Complex
- Role: Receives electrons from ubiquinone and transfers them to cytochrome c.
- Proton Pumping: Uses some of the energy from electron transfer to pump protons (H⁺) across the membrane, contributing further to the proton gradient.
Cytochrome c
- Role: A small, water-soluble electron carrier that shuttles electrons from Complex III to Complex IV.
- Mobility: Moves along the outer surface of the inner membrane.
Complex IV: Cytochrome c Oxidase
- Role: Accepts electrons from cytochrome c and transfers them to molecular oxygen (O₂), the final electron acceptor.
- Proton Pumping: Pumps additional protons across the membrane.
- Water Formation: Oxygen combines with electrons and protons to form water (H₂O).
Proton Gradient and ATP Synthase
- Proton Motive Force: The flow of protons into the intermembrane space creates a high proton concentration, known as a proton gradient.
- ATP Production: Protons flow back into the matrix through ATP synthase, which uses this flow to convert ADP and inorganic phosphate (Pi) into ATP. This process is known as chemiosmosis.
Summary: The ETC is a sequence of redox reactions that occurs in the mitochondrial membrane. Electrons from NADH and FADH₂ are transported through four complexes. As the electrons are transferred, protons are pumped across the inner mitochondrial membrane, resulting in the generation of a proton gradient. This gradient is utilized by ATP synthase to produce ATP, the energy currency of the cell, and water is formed as the final product with oxygen serving as the terminal electron acceptor.
FRQ Writing Samples & Feedback
FRQ Practice Sample 1
(a) The catabolism of glucose in glycolysis generates NADH, which carries high-energy electrons needed for ATP production later in cellular respiration. The oxidation of intermediates in glycolysis and the Krebs cycle releases these high-energy electrons, which are transported to the electron transport chain (ETC). In the ETC, the energy from these electrons is used to pump hydrogen ions (H+) across the inner mitochondrial membrane into the intermembrane space, setting up a proton gradient. This gradient drives ATP synthesis when the ions move back into the mitochondrial matrix through ATP synthase, producing ATP.
(b) Nearly all organisms perform glycolysis, indicating that this metabolic pathway originated in a common ancestor and has been passed down through evolutionary time. Glycolysis occurs in the cytosol, which means that even organisms without complex internal structures or organelles can perform it. The fact that glycolysis functions under anaerobic conditions also supports its ancient origin, as early Earth had low atmospheric oxygen levels.
(c) The energy released from the hydrolysis of 30 moles of ATP is 219 kcal. The efficiency of ATP production from one mole of glucose is approximately 31.9%, as calculated by dividing the 219 kcal of energy captured by the total 686 kcal available from complete oxidation of glucose. The remaining energy is released as heat, which contributes to maintaining body temperature.
(d) A possible scientific question could be: “Was the mitochondrion originally a free-living prokaryotic cell that formed a symbiotic relationship with a larger cell, eventually becoming an organelle within eukaryotic cells?” This theory, called the Endosymbiotic Theory, is supported by the presence of enzymes in mitochondria that are also found in prokaryotes, suggesting a shared evolutionary origin.
Teacher Feedback
(a) Points awarded: 1
More specific language about the role of NADH and how it contributes to ATP production in the ETC is needed. Explicitly mention that NADH and FADH2 donate high-energy electrons to the ETC, resulting in ATP production.
Good explanation of hydrogen ions, but remember they are in the intermembrane space, not outside the cell.
(b) Points awarded: 2
Great work explaining the ancient and universal nature of glycolysis, but be sure to connect this to the inheritance from a common ancestor.
(c) Points awarded: 1
Correct value for energy available from ATP hydrolysis, but efficiency calculation needs more detail. Excess energy is released as heat, not stored for later use.
(d) Points awarded: 1
Good response overall, but consider adding more details from the question to better frame your answer.
FRQ Practice Sample 2
(a) The catabolism of glucose in glycolysis and pyruvate oxidation produces electron carriers, NADH and FADH2, which are used in the ETC to generate ATP. The Krebs cycle further produces NADH and FADH2, which transport high-energy electrons. In the ETC, these electrons are used to pump H+ ions into the intermembrane space, creating a gradient. This gradient then drives the synthesis of ATP as H+ ions flow through ATP synthase into the mitochondrial matrix.
(b) The fact that nearly all organisms perform glycolysis indicates that this pathway likely originated in a common ancestor, who then passed it down through evolutionary history. Glycolysis takes place in the cytosol, which means it does not require specialized organelles, allowing even simple prokaryotic cells to perform this process. Moreover, glycolysis can proceed anaerobically, supporting the idea that early Earth conditions likely favored such a pathway before atmospheric oxygen became abundant.
(c) The energy available from 30 ATP molecules is 219 kcal, giving a total efficiency of approximately 32%. Excess energy not captured as ATP is released as heat, which contributes to thermoregulation in organisms.
(d) A relevant scientific question is: “Were mitochondria once independent prokaryotic cells that entered into a mutualistic relationship with larger cells, eventually evolving into organelles?” The Endosymbiotic Theory supports this idea, noting the similarities between prokaryotic enzymes and those present in mitochondria.
Teacher Feedback
(a) Points awarded: 2
Excellent explanation of the role of electron carriers and their function in the ETC. However, be sure to explicitly state how the H+ gradient drives ATP production.
(b) Points awarded: 3
Great job explaining the evolutionary basis for glycolysis. Clear, concise, and well-supported.
(c) Points awarded: 3
Perfect response. Great job calculating efficiency and explaining the fate of excess energy.
(d) Points awarded: 1
Good use of the Endosymbiotic Theory, but try to incorporate specific details from the prompt for a more complete response.







