Chapter 1 : Motion, Forces And Energy
Physical Quantities and Measurement Techniques
Rulers and measuring cylinders are used for length and volume.
Measuring a variety of time intervals using clocks and digital timers.
Scalar
Quantities that only have magnitude.
Distance is also scalar as it has no direction.
Examples: Speed, time, mass, energy, and temperature.
Vectors
Quantities that have both magnitude and direction.
Velocity is also a vector because it is necessary to mention both its speed and direction.
Examples: Force, weight, acceleration, momentum, electric field strength, and gravitational field strength.
The Resultant Force of Two Vectors at Right Angles
Calculation Graphically

Motion
Speed is the distance traveled per unit time.
Velocity is speed in a given direction.
Acceleration is the change in velocity per unit time:

Speed = gradient of distance – time graph
Acceleration = gradient of speed – time graph
Distance traveled = area under the speed – time graph
Deceleration is negative acceleration and should be used in calculations.
Falling Object Without Air Resistance:
An object falls with the same acceleration. The speed of the falling object increases at a steady rate.
Falling Object With Air Resistance:
In a uniform gravitational field, objects experience weight and friction.
The force of air resistance increases with the speed of the falling object.
Initially, the upward air resistance isn’t high, meaning there are unbalanced forces.
As air resistance increases, it balances the downward force. When air resistance equals weight, the forces are balanced, and the object falls at a constant speed called Terminal Velocity.
Acceleration of free fall g for an object near to the surface of the Earth is approximately constant and is approx constant and is 9.8 m/s²

Mass and Weight
Mass:
A measure of the quantity of matter in an object at rest relative to the observer.
Weight:
A gravitational force on an object that has mass.
Weight is the effect of a gravitational field strength on mass.
Weight = Mass × Gravitational Field Strength
Gravitational Field Strength is force per unit mass. This is equivalent to the acceleration of free fall.
Weights (and mass) can be compared using a balance.

| Mass | Weight |
|---|---|
| The amount of matter in a body. | Due to pull of gravity on the body. |
| Has only magnitude. | Has both magnitude and direction. |
| Measured in kilograms (kg). | Measured in Newtons (N). |
| It remains constant regardless of place or location. | Changes from place to place. |
Density
Density is mass per unit volume.
where:
= density
= mass
= volume
How to Determine Density in Solids
| Regular Solid | Irregular Solid |
|---|---|
 |  |
| Measure length, width, height & multiply to find volume. | Place object into a measuring cup until it is submerged in water; the increase in water volume is the volume. |
| Place object on a balance to find its mass. | Place object on a balance to find mass. |
| | |
Which Object Floats?
Any object with a density lower than that of the liquid will float above the liquid.
If Liquid Doesn’t Mix, Does it Float?
Lower density liquids float on denser liquids if not mixed.
Forces
Forces may produce changes in the size and shape of an object.
The spring constant as force per unit extension:
 Limit of proportionality is the point beyond which the extension of an elastic object is no longer directly proportional to the force applied to it.
Limit of proportionality is the point beyond which the extension of an elastic object is no longer directly proportional to the force applied to it.
Resultant Force of two forces that act in a straight line in the same way is found by just adding them together.

An object either remains at rest or continues in a straight line at constant speed unless acted on by a resultant force.
A resultant force may change the velocity of an object by changing the direction of motion or speed.
Motion in a Circular Path Due to a Force Perpendicular to the Motion:
Speed increases as force increases, with mass and radius constant.
Radius decreases if force increases, with mass and speed constant.
An increased mass requires an increased force to keep speed and radius constant.
Solid friction is the force between two surfaces that may impede motion and produce heating.
Friction (drag) acts on an object moving through a liquid and also gas (e.g., air resistance).
Turning Effects of Forces
The moment of a force is a measure of its turning effect.
Examples: Door hinges, a seesaw, unscrewing a nut.
Moment = Force × Perpendicular Distance from the Pivot
Principle of Moments is when a body is balanced, the total clockwise moment about a point equals the total anticlockwise moment about the same point.

When there is no resultant force and no resultant moment, an object is in equilibrium.
A simple experiment to demonstrate there is no resultant moment on an object in equilibrium involves taking an object like a beam and replacing the support with supports with Newton (force) meters. The beam will be in equilibrium if both sides exert the same force.
Centre of gravity of an object is the point at which the weight of the object may be considered to act.
To find the centre of gravity of an irregularly shaped plane lamina:
Hang up the irregularly shaped object.
Suspend the shape from a location near an edge. Drop a plumb line and mark it on the object.
Suspend the shape from another location and drop a plumb line, and mark the position. The place where the lines intersect is the centre of gravity.
The position of the centre of gravity of an object affects its stability. The lower the centre, the more stable the object.

Momentum
Momentum = Mass × Velocity
Impulse = Force × Time
Principle of Conservation of Momentum: States that if two objects collide, total momentum before and after collision remains the same if there are no external forces.
Resultant Force is the change in momentum per unit time:
Energy, Work, and Power
Energy
Energy may be stored as kinetic, gravitational potential, chemical, elastic (strain), nuclear, electrostatic, and internal (thermal).
Energy Transfer
Energy is transferred between stores during events and processes. e.g.,
Transfer by Forces (Mechanical Work Done)
When a force acts on an object (e.g., pulling, pushing, stretching, etc.).
Transfer by Electrical Currents (Electric Work Done)
When charge (current) moves through a potential difference.
Transfer by Heating
When energy is transferred from a hot object to a colder one.
Transfer by Electromagnetic, Sound, and Other Waves
Energy transferred by electromagnetic waves (e.g., light).
Kinetic Energy
Energy that an object has as a result of its mass and speed.
where:
= mass
= velocity
= kinetic energy
Gravitational Potential Energy
Energy an object has due to its height in a gravitational field.
Change in GPE:
where:
= mass
= gravitational field strength
= change in height
Principle of Conservation of Energy
Energy can’t be created or destroyed; it can only be transferred from one store to another.
Sankey Diagrams
Used to represent energy transfers.
Flat end of the arrow shows energy in.
Straight arrow shows useful energy out.
Arrows bending away show waste energy. Total Energy In = Useful Energy Out + Wasted Energy
Total Energy In = Useful Energy Out + Wasted Energy
Mechanical or electrical work done is equal to the energy transferred.
work = Force × distance
work = change in energy(J)
Energy Resources
Useful energy may be obtained, or generated from:
Chemical energy in fossil fuels & biofuels
Water – energy in waves, tides & dams
Geothermal resources
Nuclear fuel (non-renewable)
Light from the sun to generate power (solar cells)
Infrared & other electromagnetic waves from the sun to heat water (solar panels) and be a source of wind energy.
| Energy Resource | Renewable? | Advantages | Disadvantages |
|---|---|---|---|
| Fossil fuels | No | Reliable. Can produce large amounts of energy at fairly short notice. | Produces significant greenhouse gases and pollution. |
| Nuclear | No | Reliable. Produces no greenhouse gases or pollution. A large amount of energy is produced from a small amount of fuel. | Produces dangerous radioactive waste that can take thousands of years to decay. |
| Bio-fuels | Yes | The CO₂ produced while burning the fuel is balanced by the CO₂ absorbed whilst producing it. | Can take up a lot of land and consume resources that are needed for food production. |
| Wind | Yes | Produces no greenhouse gases or pollution. Land can still be used for farming. | Not reliable. Turbines can be noisy and ugly. Not everywhere is suitable. |
| Hydroelectric | Yes | Reliable and can produce a large amount of energy at short notice. Produces no pollution or greenhouse gases. | Can involve flooding large areas, destroying important wildlife habitats. |
| Tidal | Yes | The tides are very predictable, and a large amount of energy can be produced at regular intervals. | Very few suitable locations. Can cause environmental harm to estuaries and disrupt shipping. |
| Geothermal | Yes | Reliable. Geothermal stations are usually small. | Can result in the release of harmful gases from underground. Not many places are suitable. |
| Solar | Yes | Produces no greenhouse gases or pollution. Good for producing energy in remote places. | Not reliable (only works when sunny). Solar farms can use up lots of farmland. |

Radiation from the Sun is the main source of energy for all our energy resources except geothermal, nuclear, and tidal.
Energy is released by nuclear fusion in the Sun.
Research is being done to investigate how energy released by nuclear fusion can be used to produce electric energy on a large scale.
The ratio of useful energy/power output from a system to total energy/power input is:
Power is work done per unit time and energy transferred per unit time.
(a)
(b)
Pressure
Pressure is force per unit area.
In real life, pressure is seen in any force exerted:
Pushing a door
Standing on the floor
Nail/thumb pin
Pressure unit is Pascals (Pa).
Pressure in Liquid
Pressure in liquid is exerted from all directions.
Force acts at 90° to the surface of the object.
The formula for pressure in liquid is:
where:
= liquid density
= gravitational field
= change in height
Chapter 2: Thermal Physics
Kinetic Particle Model of Matter


| State | Solid | Liquid | Gas |
|---|---|---|---|
| Density | High | Medium | Low |
| Arrangement of particles | Regular pattern | Randomly arranged | Randomly arranged |
| Movement of particles | Vibrate around a fixed position | Move around each other | Move quickly in all directions |
| Energy of particles | Low energy | Greater energy | Highest energy |
| 2D diagram |  |  |  |
The Absolute Zero
Particles move due to energy from the surrounding temperature, but at some point, there is a temperature where particles no longer move. This is the absolute zero (-273°C).
Pressure is caused by the collision of gas particles onto the walls of its container, with forces exerted by particles colliding with surfaces.
(Force per unit area)
Brownian motion is the random movement of particles in a liquid/gas produced by large numbers of collisions with smaller particles.
Brownian motion can only be seen under a microscope, and even then, you can only see particles like smoke but not the smaller atoms and molecules.
The light, fast-moving atoms and molecules collide with larger microscopic particles.
Gases and Absolute Temperature
The Kelvin temperature scale begins at absolute zero.
0K is equal to -273°C.
1K increase is the same as a 1°C increase.
It is impossible for temperature to be lower than 0K; it can never be negative.
Converting Kelvin to °C:
A change in temperature (increase) will cause pressure to increase.
A change in volume (increase) will cause pressure to increase.
Thermal Properties and Temperature
Thermal Expansion
When materials are heated, they expand.
The space taken up by molecules increases; the molecules themselves don’t increase in size.

Everyday Application and Consequences of Thermal Expansion
Thermometers rely on the expansion of liquid to measure temperature.
Bimetallic strip that bends up when heated and closes the circuit.
Consequences of Expansion:
Metal railway tracks, road surfaces, and bridges have gaps built in to account for expansion.
A rise in temperature of an object increases its internal energy.
The rise in temperature of an object causes an increase in the average kinetic energies of all particles in the object.
Specific heat capacity is the energy required per unit mass per unit temperature. where:
is the change in energy
is mass
is the change in temperature
Experiment to measure specific heat capacity

Melting:
When solid turns to liquid.
Boiling:
When vapor pressure equals liquid pressure; no internal temperature rise.
Ice melts at 0°C, and water boils at 100°C.
Boiling happens throughout the liquid at a set temperature, while evaporation happens only at the surface and at any temperature.
Condensation: (gas → liquid)
Particles lose kinetic energy (KE) and come closer and become slower.
Solidification: (liquid → solid)
Particles lose more KE, barely move and only vibrate in a fixed position.
Evaporation:
The escape of more energetic particles from the surface of the liquid.
Higher temperature = more evaporation.
Higher surface area = more evaporation.
Higher air movement = more evaporation.
Evaporation causes cooling of a liquid:
Particles at the surface of the liquid gain energy and change into vapor, so all high-energy (high temp) particles vaporize, leaving behind the low-energy cool particles.
Transfer of Thermal Energy
Conduction
Thermal conduction occurs when two solids of different temperatures come in contact with one another, transferring thermal energy from the hot object to the cold object.
Metals are the best conductors because of the high number of free-moving electrons. Atoms need to vibrate and collide to pass the energy.
Conduction is bad in liquids and gases due to particles being further away, meaning the vibration can’t be passed.
Conductors tend to be metal; better conductors have delocalized electrons to transfer energy.

Convection
Convection is the main mode for heat to travel through liquids and gases.
Convection can’t happen in solids.
When a liquid or gas is heated, it becomes hotter than the surroundings. The hot liquid/gas rises, and the cooler liquid/gas will sink and take its place, repeating the process. This is called a convection current.

Radiation
Thermal radiation is infrared radiation, and all objects emit this radiation. This radiation doesn’t require a medium.
For an object to be at constant temperature, it needs to transfer energy away from the object at the same rate that it receives energy.
Different surfaces radiate and reflect heat differently.
Example:

If an object receives energy at a rate higher than the loss, the object’s temperature will increase (and vice versa).
Greenhouse Effect
The temperature of the Earth is controlled by incoming and emitted radiation.
Infrared from the Sun is:
Reflected back to space.
Absorbed by the Earth’s atmosphere/surface.
Emitted from the Earth’s atmosphere/surface to space.
Radiated heat is directly proportional to the surface area and temperature of the object.
Consequences of Thermal Energy:
Heating objects with kitchen pans

A fire burning wood/coal

Heating a room by convection

A radiator in a car

Experiments to Distinguish Good & Bad Absorbers and Emitters

Chapter 3: Waves
General Properties of Waves
Wave:
A wave transfers energy without transferring matter.
Wave Motions:
Wave motions are oscillations and vibrations.
Examples: Ropes, strings, and water waves.

Frequency
Number of waves passing a point per second, measured in Hertz (Hz).
Wave Speed
Distance traveled by wave per second.
Frequency × wavelength
Wave Fronts
Imaginary surface corresponding to points of waves that vibrate in unison.

Reflection
Wave hits boundary between two media & doesn’t pass through, and stays in the original medium.
Angle of incidence = angle of reflection.
Refraction
A wave passes a boundary between two transparent media and undergoes a change in speed. A wave refracts and undergoes a change in wavelength and direction.
If wave slows down, waves bunch up & decreases.
If wave speeds up, waves spread out & increases.
Diffraction
When waves pass a narrow gap, the waves spread out; this is called diffraction.
If the gap is smaller, diffraction is more prominent.

Transverse Waves:

Wave vibration is perpendicular to wave propagation.
Electromagnetic radiation, water waves, and seismic (S) secondary waves can be modeled as transverse.
Longitudinal Waves:

Wave vibration is parallel to wave propagation.
Sound and seismic (P) primary waves can be modeled as longitudinal waves.
Using a Ripple Tank
Reflection at a plane surface.

Refraction due to a change of speed caused by a change in depth.

Light
Reflection of Light

Normal:
line that is perpendicular to plane (at 90°)
Angle of incidence:
angle of wave approaching plane
Angle of reflection:
angle of wave leaving plane
Angle of incidence = Angle of reflection
A virtual image is formed by the divergence of rays from the image, and can’t be projected onto a piece of paper (rays don’t go through the image).

Safety:
ray box can burn
Don’t look into light
keep liquid away from items
Control variables:
distance of ray box, wavelength, width

Refraction Of Light
Refractive index, n, is the ratio of speeds of a wave in two different regions.
where:
c: Critical angle

Critical angle:
The angle of incidence at which the angle of refraction is 90° degrees.
Total internal reflection:
When the angle of incidence is greater than the critical angle.



Optical fibres:
technology that transmits info as light pulses along glass/plastic fiber
used by telecommunication to transmit telephone signals, internet & cable TV signals.

Lenses can be used to:
single lens for magnifying
use converging & diverging lens to correct long/short sightedness

Thin Lenses
Principal axis:
Line passing through lens centre.
Principal focus:
Point at which rays of light intersect with principal axis.
Focal length:
Distance between centre & focal point.

Converging lenses:
Parallel rays are brought to a focus called principal focus/focus point. – convex
Diverging lenses:
Parallel light rays are made to diverge (spread out) from a point. – concave

Real image:
Image formed when rays converge & project on screen.
Virtual image:
Image formed when light rays meet behind lens.
Converging Lens real image
Converging lens virtual image
Dispersion of Light
The dispersion of light occurs when white light is refracted by a glass prism. The light splits to form a spectrum of 7 colors. This is because each color has different wavelength & frequency.
Red Orange Yellow Green Blue Indigo Violet
Longest wavelength Shortest wavelength
Lowest frequency Highest frequency
A ray of single color/wavelength is called monochromatic.
Electromagnetic Spectrum
Electromagnetic spectrums have specific order based on wavelength or frequencies:
Highest wavelength & lowest frequency
![]() Radio waves: radio, TV transmissions, astronomy, RFID
Radio waves: radio, TV transmissions, astronomy, RFID
Microwaves: satellite, TV, mobile, microwave ovens, phones
Infrared: electric grills, short range communication, thermal imaging
Visible light: vision, photography, illumination
Ultraviolet: security marking, detecting fake bank notes, sterilization
X-rays: medical scanning, security scanners
Gamma rays: sterilizing food & medical equipment, cancer detection & treatment
Lowest wavelength & highest frequency
All electromagnetic waves travel at the same high speed, which is 3.0 × 10⁸ m/s and it’s approximately the same in air.
Digital Signal:
Signal represented by binary numbers
Analogue Signal:
Representation of direct copy of original source
Sound can be transmitted as both analogue & digital signals.

Harmful effects of electromagnetic waves:
Microwaves: internal heating of body cells
Infrared: skin burns
Ultraviolet: damage to surface cells & eyes – cancer
X-rays & Gamma Rays: mutation & damage to cells
Many important communication systems rely on electromagnetic radiation including:
Mobile phones & wireless internet: microwaves used because they penetrate walls & require short aerials.
Bluetooth: uses low energy radio waves or microwaves as they pass through walls but signal is weakened by doing so.
Optic fibres: (visible light/infrared) used for cable TV & high-speed broadband, visible light carries high rates of data.
Communication with artificial satellites is mainly by microwaves:
Some satellite phones use low orbit artificial satellites.
Some satellite phones & direct broadcast satellites use geostationary satellites.
Sound
Sound is produced by vibrating sources!
Sound waves are of longitudinal nature.
![]() Compression Rarefaction
Compression Rarefaction
The approximate range of frequency audible to humans is 20 Hz – 20,000 Hz.
A medium is needed to transmit sound waves (vibrating particles, so a medium is needed).
The speed of sound in air is approximately 330-350 m/s.
In general, sound travels faster in solids than in liquids and gases, and faster in liquids than in gases.
Echoes are a reflection of sound waves.
Ultrasound is a sound with a frequency higher than 20 kHz. It is used in non-destructive testing of material, medical scanning of soft tissue, & sonar.
Average speed =

Frequency is related to pitch
High pitch → high frequency
Low pitch → low frequency
Amplitude is related to volume
High amplitude → high volume
Low amplitude → low volume
Chapter 4: Electricity & Magnetism
Simple phenomena of magnetism
The two ends of a magnet are called poles:
North & South poles.
- The like poles repel (push each other apart).
- The unlike poles attract (move towards each other).
Magnetic materials:
- Experience force when in a magnetic field, attracted to a magnet when unmagnetised.
- Can be magnetised to form a magnet.
- Only a magnet can repel another magnet (test).
Non-magnetic materials:
- Do not experience a force when placed in a magnetic field.
Permanent magnets:
- Compass – always points north due to Earth’s south pole.
- School lab experiment – permanent magnets for demo.
- Toys.
- Fridge magnets – stick magnet to back of charm.
Uses of electromagnets (temporary magnets):
- MRI scanners – used to produce diagnostics of organs.
- Speakers / Earphones – to sense/send sound waves.
- Recycling – used to separate & recycle metal from rubbish.
A magnetic field is a region in which a magnetic pole experiences a force.
Pattern & direction of magnetic field around a bar magnet:
- The closer the lines are in the field, the stronger the magnetic field.
The direction of the magnetic field always goes from North to South. Magnetic field lines can be plotted by the iron filling method:
- Place magnet below paper.
- Spread iron fillings around paper
- Let the fillings settle on the field lines.
- Place magnet on paper & draw dot at corner of magnet & Place plotting compass near dot, place another where the previous points & repeat.
Electric Circuits
Circuit diagrams:
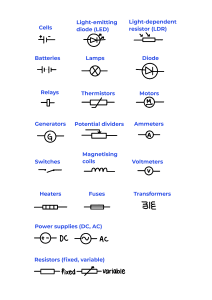
Current at any point in a series circuit is the same:
- The sum of the currents entering a junction in a parallel circuit is equal to the sum of current leaving.
- Total P.d. (potential difference) across the components in a series circuit is equal to the sum of the individual P.d.s across each component.
- P.d. across an arrangement of parallel resistances is the same as P.d. across one branch in the arrangement of parallel resistances.
Constructing Series & Parallel:
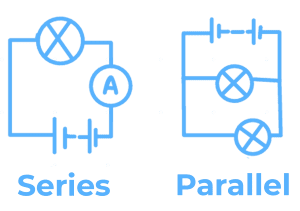
Combined e.m.f. in Series = Sum of all sources.
Combined resistance in Series =
For a parallel circuit, the current from the source is larger than the current in each branch.
The sum of the currents into the junction is the same as the sum of currents exiting the junction.
Combined resistance of two resistors in parallel is less than that of either resistor by itself:
The advantage of connecting lamps in parallel is that you are able to switch on/off separate lights; even if one lamp is broken, the rest will work.
The P.d. (potential difference) across a conductor increases as its resistance increases for constant current.
A variable P.d. works with 2 resistors.
The input voltage is applied across resistors, and output is taken across one of the resistors:
Electrical Quantities
There are positive & negative charges. Charge is measured in coulombs.
- Like charges repel; opposite charges attract.
- An electric field is a region in which electric charge experiences a force.
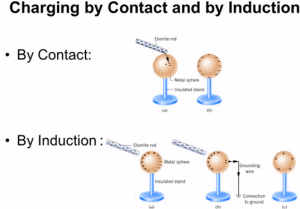
Experiment to Show Production of Electrostatic Charges:
The direction of an electric field at a point is the direction of the force on a positive charge at that point.
Charging of solids by friction involves only a transfer of negative charge (electrons).
Electric Current is related to the flow of charge.
Electric current is charge passing a point per unit time.
Where:
= Current
= Charge
= Time
Use of ammeters is to measure current.
Use of voltmeters is to measure volts.

Electrical conduction in metals happens by allowing free electrons to move between atoms.
Conventional current is from positive to negative, and the flow of free electrons is from negative to positive.
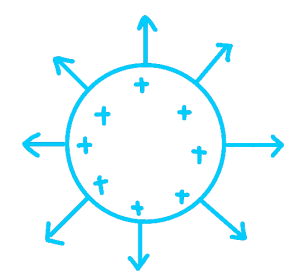
Simple electric field patterns, Direction of field:
Around a point charge:
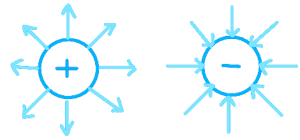
Around a charged conducting sphere:
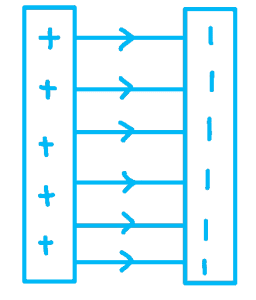
Between two oppositely charged parallel conducting plates:
Electrical Conductors and Insulators:

Electron model:
Direct Current (DC):
When current flows in one constant direction (e.g., batteries, solar cells).
Alternating Current (AC):
When current periodically inverts its direction (e.g., electrical appliances, home sockets).
Electromotive Force (e.m.f.) is the electrical work done by a source in moving unit charge around a circuit.
Where:
= Work
= Charge
Potential Difference (P.d.) is the work done by a unit charge passing through a component.
Where:
= Work done
= Charge
Resistance is how difficult it is for current to pass through a component, measured in Ohms (Ω).
Resistance is directly proportional to length.
Resistance is inversely proportional to cross-sectional area.
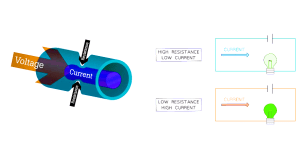
Electrical Quantities (Continued…)
Electric circuits transfer energy from a source of electrical energy, such as an electrical cell or mains supply, to the circuit components and then to the surroundings.
Electrical Power = Current × Voltage
Electrical Energy = Current × Voltage × Time
Kilowatt per hour (kWh) is equal to the energy converted by a 1kW device for one hour.
Electrical Safety
Hazards of:
- Damaged insulation: electrocution
- Overheating cables: burns & short circuit
- Damp conditions: electrocution, short circuit
- Excess current from plug overload: fires, short circuit
A mains circuit consists of a live wire (line wire), a neutral wire, and an earth wire. A switch should always be connected to the live wire so when it’s switched off, no current flows through the appliance to prevent electrocution and overloading.
A fuse is a thin wire that heats up and melts when an excess current flows through it. A fuse has a rating, and this is the maximum current that can flow through it without melting the wire.
Choose a rating higher than the current.
The outer case of an electrical appliance must be non-conducting or earthed to prevent electric shocks.
A fuse without an earth wire protects the circuit and the cabling for a double-insulated appliance.
Electromagnetic Effects
A conductor moving across a magnetic field or changing the magnetic field linking with a conductor can induce an e.m.f (electromotive force) on the conductor.
The direction of an e.m.f opposes the change causing it.
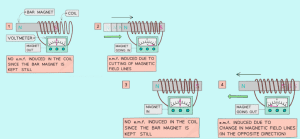
Experiment to demonstrate the electromagnetic induction
Fleming’s Right Hand Rule:

Used to find the direction of induced current when a conductor moves in field.
Factors affecting magnitude of induced e.m.f.:
- Speed of wire, coil, or magnet movement
- Number of turns on the coils of wire
- The size of coils (larger coil = larger P.d.)
- Strength of the magnetic field
Alternating Current Generator
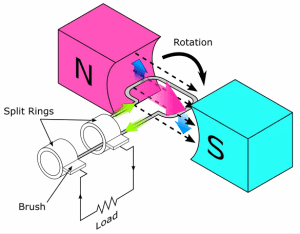
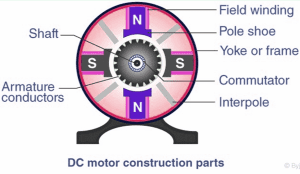
Direct Current Motor:
A current-carrying coil in a magnetic field may experience a turning effect and that the turning effects is increased by increasing:
- the number of turns on the coil
- the current
- the strength of the magnetic field
Magnetic Effect of a Current:
Current in straight wires:

Current in solenoids:

The magnetic field created by the solenoid is much stronger than that created by a straight wire or a flat circular coil.
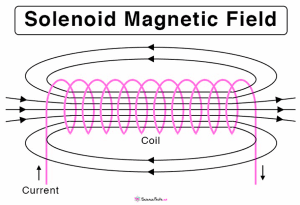
Electromagnetic effects
Electromagnets are used in relays. A relay is a device that uses a low current circuit to switch a high current circuit on or off.
- When the switch in the low current circuit is closed, it turns the electromagnet on, which attracts an iron armature.
- armature pivots and closes the switch contacts in the high current circuit.
- When low current opens, the electromagnet stops pulling the armature, and the high current circuit is broken again.
Effect on the magnetic field around straight wires and solenoid of changing the magnitude & direction of the current.
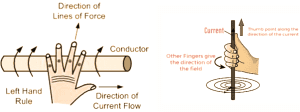
A current-carrying conductor will only experience a force if current is perpendicular to the direction of magnetic field lines. If the current or direction of the field is reversed, the N/S poles are also reversed.
Transformer Efficiency
If a transformer is 100% efficient,
Where:
is Voltage (Volts)
is Current (Amps)
or…
is Output Power produced in the Secondary Coil (Watts).
High Voltage Transmissions:
- Used to increase P.d. before being transmitted to the national grid.
- Used to lower high voltage electricity used in power lines.
- Used in adapters to lower mains voltage.
Advantages:
- Reduce energy loss = less current, less heat in wires.
- Energy loss = or
Transformers
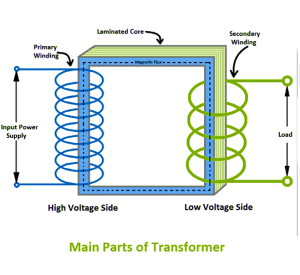
A transformer is an electrical device that can increase or decrease the potential difference of alternating currents.
- This can be done using the generator effect.
A basic transformer consists of:
- Primary coil
- Secondary coil
- Soft iron core
Operation of a transformer:
- Alternating current is supplied to the primary coil.
- The current is continually changing direction, producing a changing magnetic field around the primary coil.
- The iron core is easily magnetized, so the field passes through it.
- This creates a changing field inside the secondary coil, causing it to induce a potential difference.
- Since the magnetic field is changing, the P.d. induced will be alternating.
- The alternating P.d. has the same frequency as the current to the primary coil.
- The secondary coil is part of a complete circuit and causes AC flow.
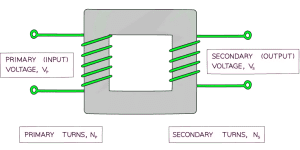
Primary coil – first coil
Secondary coil – second coil
Step-up transformer increases P.d. of the power source (more turns on secondary coil than primary).
Step-down transformer decreases P.d. of the power source (fewer turns on secondary coil than primary).
Transformer Calculations
Output potential difference (voltage) depends on:
- Number of turns on &
- Input potential difference
Chapter 5: Nuclear Physics
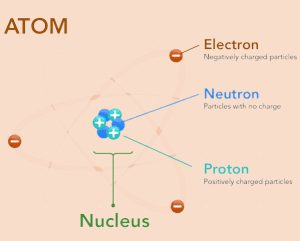
The Nuclear Model of the Atom
The Atom
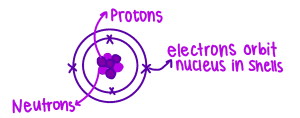
Scattering alpha (α) particles by a thin sheet of metal supports the nuclear model of the atom as it proves:
- Small nucleus surrounded by empty space
- Nucleus contains most of the atom’s mass
- Nucleus is positively charged
Atoms may form positive ions by losing electrons or form negative ions by gaining electrons.
The nucleus is composed of neutrons and protons.
Relative charge of:
Electrons = -1
Protons = +1
Neutrons = 0
Proton number (atomic number)
Nucleon number (mass number)
To find the number of neutrons, subtract from .
Charge of the nucleus is given by the number of its protons ().
Mass number = Total nucleon number.
Nuclide notation =
- Isotope is two or more species of an atom with the same atomic number () but different atomic mass ().
- An element may have more than 1 isotope.
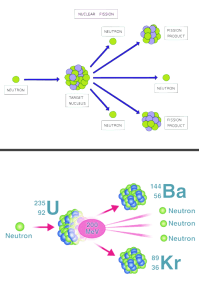
Nuclear Fission
Nuclear fission is the splitting of a large unstable nucleus into two smaller nuclei.
Products of fission move away very quickly:
- nuclear potential energy to kinetic energy.
- mass of products is less than the original nucleus, causing the remaining mass to be converted into energy that is released.
Nuclide Equation for Fission:
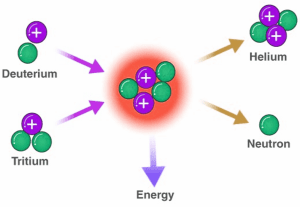
Nuclear Fusion
Nuclear fusion is when two light nuclei join to form a heavier nucleus.
This process requires high temperatures to maintain, making nuclear fusion hard to reproduce.
Energy produced during nuclear fusion comes from a small amount of particle mass being converted into energy:
Where:
= Energy released in fusion (joules)
= Mass converted to energy (in kg)
= Speed of light (m/s)
mass of the product is less than the mass of the two original nuclei
this is because remaining mass has been converted into energy.
Nuclide equation for fusion:
– Deuterium (hydrogen isotope)
– Hydrogen
– Helium
Radioactivity
Detection of Radioactivity
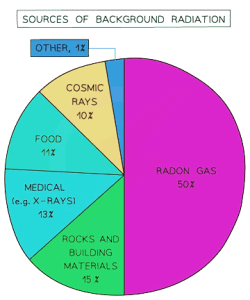
Background radiation is found in small quantities all around us and originates from natural sources.
Sources that contribute to background radiation:
- Radon gas (in the air)
- Rocks and buildings
- Food and drink
- Cosmic rays
Ionising nuclear radiation can be measured using a detector connected to a counter.
- Count rate is measured in counts per second or counts per minute.
- Background radiation count rate is subtracted from each measurement so the actual count rate is calculated.
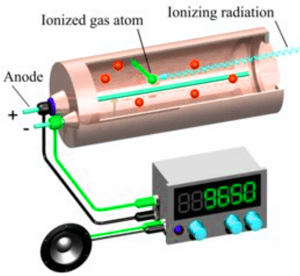
Three Types of Nuclear Emission
The emission of radiation from a nucleus is spontaneous and random in direction.
The three types of emissions from the nucleus are:
#1: Alpha (α) particles (deflected by magnetic fields)
- Composed of 2 protons and 2 neutrons (e.g., helium nucleus) with a positive charge.
- Highly ionising due to double positive charge and large mass.
- Ionising range of 3-5 cm.
- Low penetration; cannot penetrate too far through matter.
#2: Beta (β) particles (deflected by magnetic fields)
- Fast-moving electrons.
- Moderate ionising power; range of about 1m.
- Penetration stopped by a few mm of aluminium.
#3: Gamma (γ) radiation (not deflected)
- Electromagnetic waves.
- Low ionisation power; infinite range.
- Penetration reduced by a few mm of lead.
The greater the charge of radiation, the more ionising it is.
The higher the kinetic energy, the more ionising it is.
Radioactive Decay
Radioactive decay is the change in an unstable nucleus that can result in the emission of alpha particles (
), beta particles (
), and/or gamma radiation (
). These changes are spontaneous and random.
Isotopes of an element may be radioactive due to an excess of neutrons in the nucleus and/or the nucleus being too heavy.
During
-decay or
-decay, the nucleus changes to that of a different element.
During Alpha decay, a completely new element is formed in the process atomic number decreases by 2, mass number decreases by 4.
During beta decay, neutron changes to proton and electron. new element is formed.
During Gamma decay, No change but lots of energy is emitted, no mass or charge.
Half-Life
Half-life of an isotope is the time taken for half the nuclei of that isotope in a sample to decay.
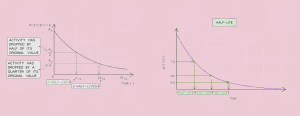
The type of radiation and half-life of an isotope determine its use for:
- Household fire (smoke) alarms
- Irradiating food to kill bacteria
- Object sterilization with gamma radiation
- Measuring object thickness
- Diagnosis and cancer treatment (gamma radiation)
Decay Equations:
Alpha decay:
Beta decay:
Gamma decay:
Safety Precautions
The effects of ionising nuclear radiations on living things include cell death, mutations, and cancer.
Radioactive material is safely stored in lead-lined boxes and kept at a safe distance from people. You must use tongs to keep away and avoid direct contact. Radioactive material is used for diagnosis, radiation medication, and radiopharmaceuticals.
Disposing of radioactive waste is done by burying it underground.
Safety precautions for all ionising radiations include reducing exposure time, increasing the distance between the source and living tissue, and using shielding to absorb radiation.
Chapter 6: Space Physics
Earth and the Solar System
The Earth is a planet that rotates on its axis, which is tilted, once in approximately 24 hours. We can observe this by the periodic cycle of day and night, the Sun and Moon’s movements, and the Earth’s spin.
The Earth orbits the Sun once every approximately 365 days. This can be seen when the Sun is furthest up in the sky (summer) and when the Sun is lower down (winter).
The average orbital speed is:
Where:
- is the average radius of orbit
- is the orbital period
It takes one month for the Moon to orbit Earth, and at different times only parts of the Moon reflect light while other parts are blocked by Earth. This is why we see Moon phases.
The Solar System contains:
- One star (the Sun)
- The eight planets: Mercury, Venus, Earth, Mars, Jupiter, Saturn, Neptune, and Uranus
- Minor planets that orbit the Sun: Dwarf planets like Pluto and asteroids in the asteroid belt
- Moons that orbit the planets
- Smaller Solar System bodies like comets and natural satellites
Note: Minor planets and comets have elliptical orbits, meaning oval-shaped orbits, and the Sun is not at the center of the elliptical orbit except when the orbit is a perfect circle.
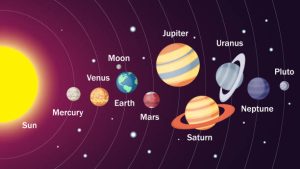
In comparison to the planets, the four closest to the Sun are rocky and small planets, while the four furthest from the Sun are gaseous and large.
Accretion Model:
The strength of the gravitational field at the surface of a planet depends on the mass of the planet, and around a planet, the strength decreases as the distance from the planet increases.
The time taken for light to travel between objects is found by taking the speed of light to be:
The Sun contains most of the mass in our solar system, so it has the strongest gravitational field strength, causing all planets to orbit the Sun.
the strength of the Sun’s gravitational field decreases and the orbital speeds of planets decrease.
An object in an elliptical orbit travels faster when closer to the Sun. This is because it loses gravitational potential energy and gains kinetic energy as it approaches the Sun, causing the object to speed up. This speed increase creates the slingshot effect when it moves away and its orbit slows.
Stars and the universe
The Sun is a star of medium size consisting mostly of hydrogen and helium. It radiates most of its energy in the infrared, visible, and ultraviolet regions of the electromagnetic spectrum.
Stars are powered by nuclear reactions that release energy, and in stable stars, the nuclear reactions involve the fusion of hydrogen into helium.
Galaxies are made up of many billions of stars. The Sun is a star in the galaxy called the Milky Way. Other stars that make up the Milky Way are much further away from Earth than the Sun.
Astronomical distances can be measured in light-years, where one light-year is the distance traveled (in vacuum) by light in one year.
1 light year = m
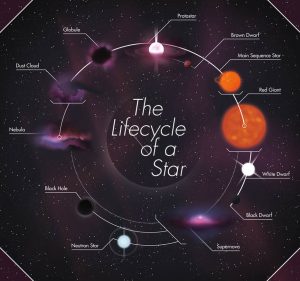
The Life Cycle of a Star
- A star is formed from an interstellar cloud of gas and dust that contain hydrogen.
- A protostar is an interstellar cloud collapsing and increasing in temperature due to internal gravitational attraction.
- Protostar becomes stable when the inward force of gravitational attraction is balanced by the outward force due to high internal temperature.
- All stars eventually run out of hydrogen as fuel for nuclear reactions.
- Most stars expand to form red giants, and more massive stars expand to form red supergiants when most of their hydrogen is converted to helium.
- A red giant from a less massive star forms a planetary nebula with a white dwarf at its center.
- A red supergiant explodes as a supernova, forming a nebula containing hydrogen and new, heavier elements, leaving behind a neutron star or black hole at its center.
- The nebula from the supernova may form new stars with orbiting planets.
The Milky Way is one of many billions of galaxies making up the Universe, with a diameter of approximately 100,000 light-years.
Redshift is an increase in the observed wavelength of electromagnetic radiation emitted from receding stars and galaxies.
The light emitted from distant galaxies appears redshifted compared with light emitted on Earth.
Redshift in light from distant galaxies is evidence that the Universe is expanding and supports the Big Bang theory.
Microwave radiation of a specific frequency is observed at all points in space around us and is known as CMBR.
CMBR was produced slightly after the Universe was formed and expanded into the microwave region of the electromagnetic spectrum as the Universe expanded.
Speed (v) at which a galaxy is moving away from Earth can be found from the change in wavelength of the galaxy’s starlight due to redshift.
The distance (d) of a far galaxy can be found using the brightness of a supernova in that galaxy.
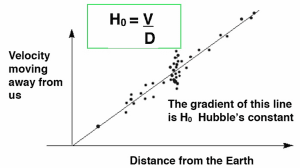
The Hubble Constant (H₀) is the speed at which the galaxy is moving away from Earth.
The current estimate for is per second.
represents the estimated age of the Universe, as all matter in the Universe was present at a single point.







