Introduction
Understanding the relationship between pressure, temperature, and volume is fundamental in the study of gases within both AP Physics and AP Chemistry. These concepts are not only essential for academic success but also have practical applications in everyday life and various scientific fields. This study guide aims to break down these concepts, provide clear explanations, and offer effective study strategies to help you excel in your next test.
Revisiting Pressure
Pressure (P) is a measure of the force exerted per unit area. It is commonly measured in atmospheres (atm) or pascals (Pa). The formula for pressure is:
- P = Pressure
- F = Force applied
- A = Surface area
Key Points:
- Greater Force, Greater Pressure: Increasing the force applied to an area increases the pressure.
- Larger Surface Area, Lower Pressure: Increasing the surface area over which the force is applied decreases the pressure.
Example:
If a force of 10 N is applied over an area of 2 m², the pressure is:
Temperature and Kinetic Energy 
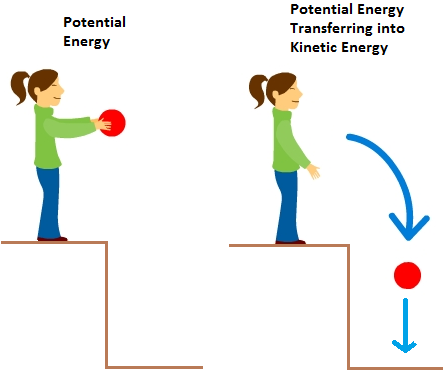
Temperature is a measure of the average kinetic energy of the particles in a substance. As temperature increases, particles move faster and collide more frequently and energetically.
Key Definitions:
- Thermal Energy: The energy that comes from the temperature of matter. It is directly related to the kinetic energy of the particles.
- Kinetic Energy (KE): The energy that an object possesses due to its motion. In gases, it relates to the motion of molecules.
- Root Mean Square Speed (RMS Speed): A measure of the average speed of gas molecules, calculated using the Boltzmann distribution.
Key Points:
- Thermal Energy is Kinetic Energy: In gases, thermal energy is a form of kinetic energy related to the motion of molecules.
- Boltzmann Distribution: Describes the distribution of speeds among molecules in a gas, indicating that as temperature increases, more molecules have higher speeds.
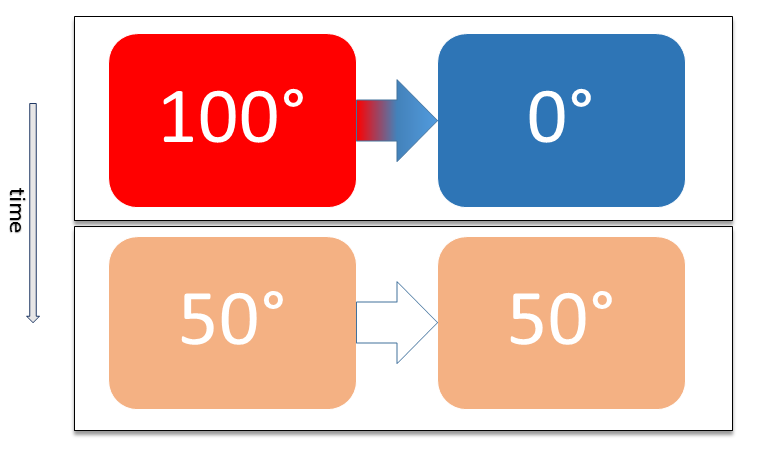
Thermal Equilibrium and Heat Transfer
Thermal Equilibrium: A state where two objects in contact with each other have the same temperature, resulting in no net flow of heat between them.
Key Points:
- Heat (Q): The transfer of thermal energy from a hotter object to a cooler one.
- Temperature (T): A measure of the average kinetic energy of particles; it dictates the direction of heat transfer.
Differences Between Heat and Temperature:
| Heat | Temperature |
|---|---|
| Energy in transit due to a temperature difference | Measure of the average kinetic energy of particles |
| Measured in joules (J) or calories (cal) | Measured in degrees Celsius (°C) or Fahrenheit (°F) |
| Transferred via conduction, convection, or radiation | Intrinsic property of an object |
| Flow from higher to lower temperature | Indicates thermal energy content |
Key Points:
- Heat is Energy Transfer: Heat is not a property of an object but rather energy moving between objects.
- Temperature is a Property: Temperature is an intrinsic measure of thermal energy within an object.
Examples:
- Ice Melting: Heat flows from the warmer room to the colder ice, causing it to melt.
- Heated Car Seat: Heat flows from the seat’s heating element to your body, making the seat warm.
The Ideal Gas Law
The Ideal Gas Law is a fundamental equation that relates the pressure, volume, temperature, and number of moles of an ideal gas. It combines several individual gas laws into one comprehensive formula.
Key Gas Laws:
- Charles’ Law:
(At constant pressure, volume increases with temperature) - Gay-Lussac’s Law:
(At constant volume, pressure increases with temperature) - Avogadro’s Law:
(At constant temperature and pressure, volume increases with the number of moles) - Boyle’s Law:
(At constant temperature, pressure decreases as volume increases)
Ideal Gas Law Formula:
- P = Pressure (atm or Pa)
- V = Volume (L or m³)
- n = Number of moles
- R = Ideal Gas Constant (0.0821 L·atm/mol·K or 8.314 J/mol·K)
- T = Temperature (K)
Applications:
- Predicting Gas Behavior: Calculate changes in one variable when others are altered.
- Stoichiometry in Reactions: Relate gas volumes to reactant and product amounts.
Example:
Calculate the pressure of 2 moles of an ideal gas occupying 10 liters at 300 K.
Assumptions and Limitations:
- Assumptions:
- Gas particles have negligible volume.
- No intermolecular forces between gas particles.
- Collisions between gas particles and container walls are perfectly elastic.
- Limitations:
- Real Gases: Deviate from ideal behavior at high pressures and low temperatures.
- Intermolecular Forces: Significant at high pressures and low temperatures, affecting pressure and volume.
- Molecular Volume: Non-negligible at high pressures, affecting volume calculations.
Van der Waals Equation:
An adjusted version of the Ideal Gas Law that accounts for molecular volume and intermolecular forces:
= Measure of intermolecular forces
= Measure of molecular volume
= Molar volume
Note: The Van der Waals equation is not typically required for the AP exams but is useful for understanding real gas behavior.
Review Questions and Detailed Answers
Question 1: How does increasing the temperature of a gas in a closed container affect its pressure?
Answer:
Increasing the temperature of a gas in a closed container increases the kinetic energy of the gas molecules. As a result, the molecules move faster and collide with the container walls more frequently and with greater force. According to Gay-Lussac’s Law and the Ideal Gas Law, this leads to an increase in pressure.
Formula:
Example Calculation:
If the temperature of a gas increases from 300 K to 600 K while the volume and number of moles remain constant, the pressure will also double.
Question 2: Explain the difference between heat and temperature with examples.
Answer:
Heat:
- Definition: Energy transferred from one body to another due to a temperature difference.
- Measured In: Joules (J) or calories (cal).
- Example: Heating a pot of water on a stove transfers heat from the burner to the water, increasing its thermal energy.
Temperature:
- Definition: A measure of the average kinetic energy of the particles in a substance.
- Measured In: Degrees Celsius (°C) or Fahrenheit (°F).
- Example: A thermometer reading indicates the temperature of the air, reflecting the average motion of air molecules.
Key Difference:
Heat is the energy in transit, while temperature is a measure of the energy within a substance.
Question 3: A gas occupies 5 liters at 2 atm and 300 K. What will be its volume at 2 atm and 600 K?
Answer:
Using Charles’ Law (
):
Explanation:
Doubling the temperature at constant pressure doubles the volume of the gas.
Related Terms
Boyle’s Law
Definition: At constant temperature, the volume of a gas is inversely proportional to its pressure.
Significance: Demonstrates how pressure and volume interact inversely in a confined gas system.
Charles’ Law
Definition: At constant pressure, the volume of a gas is directly proportional to its absolute temperature.
Significance: Explains how temperature affects the volume of a gas.
Avogadro’s Law
Definition: At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles.
Significance: Relates the amount of gas to its volume, foundational for understanding gas stoichiometry.
Gay-Lussac’s Law
Definition: At constant volume, the pressure of a gas is directly proportional to its absolute temperature.
Significance: Shows the relationship between temperature and pressure in a fixed volume.
Root Mean Square Speed (RMS Speed)
Definition: A measure of the average speed of gas molecules, calculated using the Boltzmann distribution.
Formula:
- R = Universal gas constant
- T = Temperature in Kelvin
- M = Molar mass of the gas
Significance: Helps in understanding the distribution of molecular speeds in a gas.
Quipus
Definition: A system of knotted strings used by the Incas for record-keeping and communication.
Significance: Demonstrates the Incan method of managing large-scale administrative tasks without a written language.
Common Mistakes and How to Avoid Them
Understanding gas laws can be challenging, but avoiding common mistakes can enhance your comprehension and performance in exams. Here are some prevalent pitfalls and strategies to overcome them:
1. Confusing Heat and Temperature
Mistake:
- Equating heat with temperature or using them interchangeably.
Solution:
- Clear Definitions: Remember that heat is energy transfer, while temperature is a measure of internal energy.
- Contextual Usage: Use heat when discussing energy transfer and temperature when describing the state of an object.
2. Incorrect Unit Conversions
Mistake:
- Failing to convert units properly, especially temperature (Celsius to Kelvin).
Solution:
- Always Convert to Kelvin: For gas law calculations, ensure temperature is in Kelvin.
- Check Units: Verify that all units are consistent (e.g., pressure in atm, volume in liters).
3. Misapplying Gas Laws
Mistake:
- Using the wrong gas law for a given problem or not considering all variables.
Solution:
- Identify Variables: Determine which variables are constant and which are changing.
- Select Appropriate Law: Choose the gas law that relates the known and unknown variables.
4. Ignoring Real Gas Behavior
Mistake:
- Assuming all gases behave ideally under all conditions.
Solution:
- Understand Limitations: Recognize that the Ideal Gas Law works best at high temperatures and low pressures.
- Mention Deviations: In exams, acknowledge when real gas behavior might deviate from ideal predictions.
5. Calculation Errors
Mistake:
- Simple arithmetic or algebraic mistakes during calculations.
Solution:
- Double-Check Work: Review each step of your calculations for accuracy.
- Practice Regularly: The more problems you solve, the fewer mistakes you’ll make.
Study Tips: Mastering Gas Laws
Excelling in the Pressure, Thermal Equilibrium, and the Ideal Gas Law section requires a strategic approach to studying and understanding complex relationships between variables. Here are some effective study strategies to help you master these topics:
1. Create Flashcards for Key Terms and Formulas
- Purpose: Reinforce your memory of important definitions, laws, and formulas.
- How to Use: Write the term or formula on one side and the definition or explanation on the other. Regularly review and quiz yourself.
2. Develop Comparative Charts
- Purpose: Understand the similarities and differences between various gas laws.
- How to Use: Create charts that outline key aspects of Boyle’s Law, Charles’ Law, Avogadro’s Law, and Gay-Lussac’s Law.
3. Practice Deriving the Ideal Gas Law
- Purpose: Gain a deeper understanding of how the gas laws combine to form the Ideal Gas Law.
- How to Use: Start with the four gas laws and combine them algebraically to derive
.
4. Solve a Variety of Problems
- Purpose: Apply theoretical knowledge to practical scenarios, enhancing problem-solving skills.
- How to Use: Work through textbook problems, past AP exam questions, and online practice exercises.
5. Use Visual Aids
- Purpose: Enhance understanding through visual representation of concepts.
- How to Use: Draw diagrams of gas molecules in containers, graph relationships between variables, and visualize kinetic energy distributions.
6. Teach the Material to Someone Else
- Purpose: Solidify your understanding by explaining concepts to others.
- How to Use: Study groups or teaching a friend can help reinforce your knowledge and identify any gaps.
7. Relate Concepts to Real-Life Examples
- Purpose: Make abstract concepts more tangible and easier to remember.
- How to Use: Think about how gas laws apply to everyday situations like inflating a tire, using a syringe, or weather balloons.
8. Regularly Review and Self-Test
- Purpose: Ensure long-term retention of information.
- How to Use: Schedule regular review sessions and take self-administered quizzes to test your knowledge.
9. Understand the Assumptions Behind the Ideal Gas Law
- Purpose: Recognize when the Ideal Gas Law applies and when real gas behavior deviates.
- How to Use: Study the assumptions (negligible molecular volume, no intermolecular forces, elastic collisions) and understand their implications.
10. Use Online Resources and Tutorials
- Purpose: Access additional explanations, visualizations, and practice problems.
- How to Use: Utilize educational websites, watch tutorial videos, and engage with interactive simulations to reinforce learning.
Frequently Asked Questions (FAQs)
1. What distinguishes the Ideal Gas Law from the other gas laws?
Answer:
The Ideal Gas Law combines the four primary gas laws (Boyle’s, Charles’, Avogadro’s, and Gay-Lussac’s) into a single equation:
- Boyle’s Law: Relates pressure and volume at constant temperature.
- Charles’ Law: Relates volume and temperature at constant pressure.
- Avogadro’s Law: Relates volume and number of moles at constant temperature and pressure.
- Gay-Lussac’s Law: Relates pressure and temperature at constant volume.
Significance:
The Ideal Gas Law provides a comprehensive relationship between pressure, volume, temperature, and the number of moles of a gas, allowing for more complex calculations and predictions about gas behavior under various conditions.
2. Why do real gases deviate from ideal behavior at high pressures and low temperatures?
Answer:
Real gases deviate from ideal behavior primarily due to:
- Molecular Volume: At high pressures, the volume of gas molecules becomes significant compared to the container’s volume, violating the assumption of negligible molecular size.
- Intermolecular Forces: At low temperatures, attractive forces between gas molecules become more pronounced, affecting pressure and volume as molecules are drawn closer together.
Implications:
These deviations necessitate the use of more complex equations, like the Van der Waals equation, to accurately describe real gas behavior under these conditions.
3. How does the root mean square speed (RMS speed) relate to temperature?
Answer:
The RMS speed is a measure of the average speed of gas molecules and is directly related to the temperature of the gas. As temperature increases, the RMS speed increases, indicating that gas molecules move faster on average.
Formula:
vrms
- R = Universal gas constant
- T = Temperature in Kelvin
- M = Molar mass of the gas
Significance:
RMS speed helps in understanding the kinetic energy distribution and the behavior of gas molecules at different temperatures.
Conclusion
Mastering Pressure, Thermal Equilibrium, and the Ideal Gas Law is essential for excelling in both AP Physics and AP Chemistry. These concepts form the foundation for understanding more complex topics in thermodynamics, kinetic theory, and real-world applications involving gases. By comprehensively studying these topics, practicing problem-solving, and utilizing effective study strategies, you can confidently tackle related questions in your exams and apply this knowledge in practical scenarios.
Key Takeaways:
- Pressure Fundamentals: Understand how pressure is calculated and the factors that influence it.
- Temperature and Kinetic Energy: Grasp the relationship between temperature and the kinetic energy of gas molecules.
- Thermal Equilibrium: Differentiate between heat and temperature, and understand the concept of thermal equilibrium.
- Ideal Gas Law Mastery: Know the formula
, its derivation from other gas laws, and its applications. - Real vs. Ideal Gases: Recognize the limitations of the Ideal Gas Law and when to consider real gas behavior.
- Problem-Solving Skills: Develop the ability to apply gas laws to a variety of scenarios, enhancing your analytical skills.
By integrating these concepts into your study routine and consistently practicing, you will build a strong foundation in gas laws, positioning yourself for success in your AP exams and beyond.
You got this!
References and Further Reading
- “Chemistry: The Central Science” by Brown, LeMay, Bursten, Murphy, and Woodward
- “Physics for Scientists and Engineers” by Raymond A. Serway and John W. Jewett
- “Fundamentals of Physics” by Halliday, Resnick, and Walker
- “The Kinetic Theory of Gases” by J.D. Jackson
- “Introduction to Thermodynamics: Classical and Statistical” by Richard E. Sonntag and Claus Borgnakke
- “Principles of Physical Chemistry” by Peter Atkins and Julio de Paula
- “AP Chemistry Crash Course” by Adrian Dingle
- “AP Physics 1 Essentials” by Dan Fullerton
- Khan Academy: Gas Laws https://www.khanacademy.org/science/chemistry/chem-kinetics
- Bohr Institute: Ideal Gas Law https://www.biol.brown.edu/courses/BMOLK002/FinalReview/IdealGasLaw.htm