Unit 3 Overview: Cellular Energetics
Cellular energetics is the study of how cells produce, distribute, and utilize energy. Energy is crucial for cellular functions, powering everything from metabolism to growth and repair. In this unit, we will explore how cells create energy through cellular respiration and photosynthesis, the role of enzymes in speeding up essential chemical reactions, and much more. Let’s dive in!
The Essentials of Cellular Energetics
Cells require energy for every activity they perform. In biology, cellular energetics is largely about how cells generate ATP (adenosine triphosphate), the primary energy currency in cells, through processes like cellular respiration and photosynthesis.
Cellular Respiration
In cellular respiration, glucose is broken down through a series of reactions to produce ATP, which is used by cells to power many biological processes. This process primarily occurs in the mitochondria of eukaryotic cells, while in prokaryotes, it takes place in the cytoplasm.
Photosynthesis
Photosynthesis is the process where plants, algae, and some bacteria convert solar energy into chemical energy stored in glucose or other organic molecules. This process takes place in chloroplasts in plant cells and other photosynthetic pigments in algae and bacteria.
Both processes are crucial for the production of ATP, which powers cellular processes and helps maintain the highly ordered structure of living systems. Understanding the mechanisms of ATP production and energy use is fundamental to biology.
3.1 – 3.3: Enzymes and Their Role
Enzymes are proteins that act as biological catalysts, speeding up chemical reactions within cells. They are vital to almost all cellular processes, as they allow reactions to occur at a rate necessary for sustaining life. The activity of an enzyme is driven by its active site, a region that binds to a specific molecule known as a substrate.
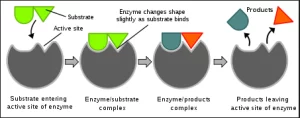
Factors Affecting Enzyme Activity
Temperature and pH: Enzymes function best within specific temperature and pH ranges. If environmental conditions fall outside these ranges, enzymes can denature, losing their shape and ability to catalyze reactions effectively.
Substrate and Product Concentration: The efficiency of an enzymatic reaction also depends on the relative concentration of substrates and products. Higher substrate concentrations generally increase the reaction rate, while excessive product levels can inhibit enzyme activity.
Competitive vs. Noncompetitive Inhibitors:
Competitive inhibitors bind to the active site, blocking substrate access and reducing enzyme activity.
Noncompetitive inhibitors bind to other sites (allosteric sites) on the enzyme, altering its shape and reducing its efficiency.
3.4: The Necessity of Energy
All living systems require a continuous input of energy to maintain their highly organized state. The second law of thermodynamics tells us that systems tend to move towards disorder, or entropy, over time. To counter this, cells constantly need energy to maintain order and continue carrying out biological functions.
Coupling of Reactions
In biological systems, reactions that release energy are often coupled with those that require energy. This energy coupling allows cells to drive processes that wouldn’t happen spontaneously, such as molecule synthesis or the movement of ions against a gradient.
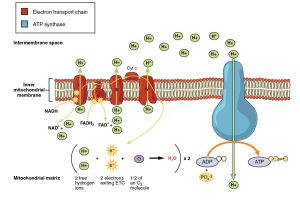
The electron transport chain (ETC) is a perfect example of a series of coupled reactions. Electrons move from molecule to molecule, releasing energy that is then used to create ATP, a process crucial for life.
3.5: Photosynthesis – Capturing Energy from the Sun
Photosynthesis captures energy from sunlight and stores it in organic molecules, primarily glucose. In prokaryotic organisms, photosynthesis was the original source of atmospheric oxygen. Later, eukaryotic organisms evolved to utilize similar pathways, facilitated by their internal chloroplasts.
Light-dependent Reactions: Chlorophyll absorbs sunlight, boosting electrons to a higher energy level in photosystems I and II. These electrons pass through the electron transport chain, generating ATP and NADPH.
Calvin Cycle: ATP and NADPH generated from light-dependent reactions power the Calvin cycle, which fixes carbon dioxide into glucose. This process occurs in the stroma of the chloroplast and forms the basis for energy storage in most plants.
3.6: Cellular Respiration and Fermentation
Organisms use cellular respiration and fermentation to generate ATP from biological macromolecules. These pathways are vital for extracting energy from food.
Fermentation is an anaerobic process that generates ATP without oxygen, often yielding lactic acid or ethanol as by-products.
Cellular Respiration is an aerobic process that occurs in the mitochondria and includes glycolysis, the Krebs cycle, and the electron transport chain.
In the ETC, electrons are passed along a chain of proteins, which eventually transfers them to a terminal electron acceptor. This creates a proton gradient across the mitochondrial membrane that drives ATP synthesis via oxidative phosphorylation.
3.7: Fitness and Adaptation
Variations at the molecular level allow organisms to better adapt to environmental changes, increasing their fitness and survival rate. The number and types of molecules within cells can change in response to different environmental stimuli, enhancing an organism’s ability to thrive.
Important Chemical Equations to Remember
Cellular Respiration: C𝑣𝑢𝑡𝑤𝑚𝑢𝑛 + O𝑢 → H𝑛O + CO𝑢
Photosynthesis: H𝑛O + CO𝑢 → C𝑣𝑢𝑡𝑤𝑚𝑢𝑛 + O𝑢
Key Players
Electron Carriers (NADH, FADH2, NADPH): Responsible for transporting electrons to various pathways to create ATP or participate in metabolic cycles like the Calvin Cycle.
ATP Synthase: The enzyme that produces ATP during chemiosmosis by adding a phosphate group to ADP. Remember that most enzymes end in “-ase” and have very specific functions.
Conclusion
Cellular energetics is all about understanding how cells produce and use energy to support life’s fundamental processes. Whether through photosynthesis, cellular respiration, or enzyme-driven chemical reactions, the way energy moves through cells is vital to the survival of every organism.
Practice Questions
What role do competitive and noncompetitive inhibitors play in regulating enzyme activity, and how do their mechanisms of action differ?
Competitive inhibitors bind to the active site, while noncompetitive inhibitors bind elsewhere, both reducing enzyme activity.