Radioactive Decay: Understanding the Basics
Introduction
Matter cannot be created or destroyed, but it can change forms. One of the most intriguing examples of this is radioactive decay, a process that demonstrates the fascinating dynamics of nuclear reactions. Other notable examples include nuclear fission and nuclear fusion. These reactions always follow the principle of conservation: nucleon number and charge are preserved.
This article explores the fundamentals of radioactive decay, its types, and its applications, providing an in-depth understanding of this essential concept in physics and chemistry.
What Is Radioactive Decay?
Radioactive decay occurs when an unstable atomic nucleus spontaneously breaks down, emitting particles and energy. This happens because the nucleus lacks the correct balance of protons and neutrons to remain stable. As a result, fragments of the nucleus are ejected, altering the material into a new form. The decaying nucleus is called the parent nuclide, while the resulting nucleus is the daughter nuclide.
One of the defining characteristics of radioactive decay is its half-life, which is the time required for half of a radioactive sample to decay. This property is fundamental to applications in fields like archaeology, medicine, and environmental science.
Types of Radioactive Decay
Radioactive decay can occur in three primary forms: alpha decay, beta decay, and gamma decay. Let’s break down each type.
Alpha Decay
Alpha decay involves the emission of an alpha particle, which consists of two protons and two neutrons (essentially the nucleus of a helium-4 atom). This type of decay reduces the atomic number by 2 and the mass number by 4.
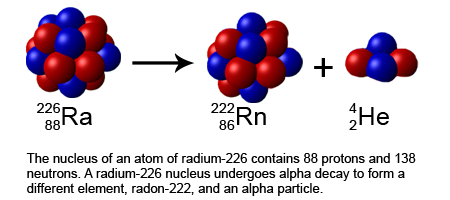
Key Points:
An alpha particle can be represented as α or .
Alpha decay occurs when the ratio of protons to neutrons in the nucleus is unstable.
This process results in a new element with a lower atomic number.
Example of Alpha Decay:
Here, uranium-238 decays into thorium-234 by emitting an alpha particle.
Beta Decay
Beta decay occurs when an atomic nucleus emits an electron or a positron. This type of decay involves three subcategories:
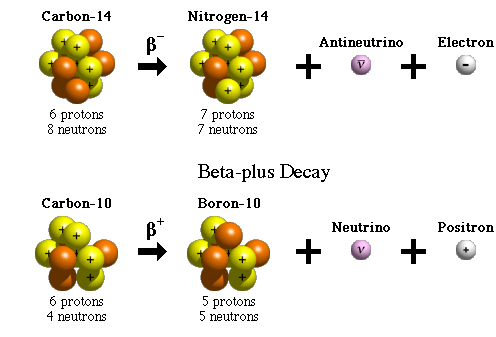
Beta-minus (B⁻) Decay: A neutron transforms into a proton, releasing an electron and an antineutrino.
Beta-plus (B⁺) Decay: A proton transforms into a neutron, emitting a positron and a neutrino.
Electron Capture: The nucleus captures an orbiting electron, converting a proton into a neutron.
Key Points:
Beta decay changes the atomic number but leaves the mass number unchanged.
It is governed by the weak nuclear force, one of the fundamental forces of nature.
Examples of Beta Decay:
Beta-minus decay:
Carbon-14 decays into nitrogen-14, emitting an electron and an antineutrino.
Beta-plus decay:
Carbon-11 decays into boron-11, emitting a positron and a neutrino.
Electron Capture:
Potassium-37 captures an electron, forming argon-37.
Gamma Decay
Unlike alpha and beta decay, gamma decay does not involve a change in the atomic number or mass number. Instead, the nucleus releases excess energy in the form of high-energy photons called gamma rays (γ).
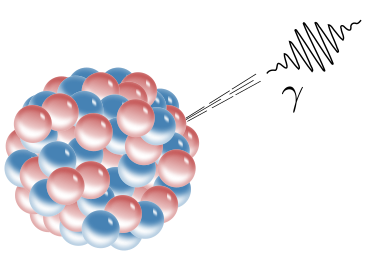
Key Points:
Gamma decay occurs when a nucleus transitions from an excited state to a lower energy state.
It is purely an energy release, with no particles being emitted or absorbed.
Gamma rays have very short wavelengths and high frequencies, making them highly penetrating.
Example of Gamma Decay:
Here, cobalt-60 emits a gamma ray to return to its ground state.
Comparing Types of Decay
| Type of Decay | Change in Atomic Number | Change in Mass Number | Emitted Particle |
|---|---|---|---|
| Alpha Decay | −2 | −4 | Alpha particle (α) |
| Beta-minus Decay | +1 | 0 | Electron (e⁻) |
| Beta-plus Decay | −1 | 0 | Positron (e⁺) |
| Gamma Decay | 0 | 0 | Gamma ray (γ) |
Practice Problems
Test your understanding of radioactive decay with these questions:
An atomic mass unit is approximately equal to the mass of a:
A) Alpha particle
B) Electron
C) Photon
D) Positron
E) Proton
Answer: E) Proton.
An alpha particle is equivalent to:
A) A helium nucleus
B) A positron
C) An electron
D) A high-energy photon
E) A deuteron
Answer: A) A helium nucleus.
During beta-minus decay, which particle is emitted from the nucleus?
A) Alpha particle
B) Beta particle
C) Gamma ray
D) Proton
E) Neutron
Answer: B) Beta particle.
When a radioactive nucleus emits a gamma ray, the number of protons and neutrons:
A) Increases by one each.
B) Decreases by one each.
C) Remains unchanged.
D) Doubles.
E) Decreases by two each.
Answer: C) Remains unchanged.
Applications of Radioactive Decay
Radioactive decay has numerous practical applications across various fields:
Medicine: Radioisotopes like iodine-131 are used in cancer treatments and diagnostic imaging.
Archaeology: Carbon-14 dating helps determine the age of ancient artifacts and fossils.
Energy: Nuclear reactors harness the energy from uranium and plutonium decay.
Environmental Science: Monitoring radioactive contamination and studying geological processes.







