2.2 Pressure, Thermal Equilibrium, and the Ideal Gas Law
Revisiting Pressure
Pressure is defined as the ratio of force applied to the surface area:
Measured in atmospheres (atm) or pascals (Pa).
Larger force = greater pressure. Larger surface area = smaller pressure.
Why is there pressure inside a container?
Gas molecules are in constant random motion, colliding with container walls and exerting a force. These microscopic collisions create the macroscopic effect of pressure.
Image Description: Gas molecules in random motion inside a container collide with walls, creating pressure.
Temperature and Kinetic Energy 🥵
Key Definitions:
Thermal Energy: Energy from increased molecular motion at higher temperatures. Closely related to kinetic energy.
Kinetic Energy: Energy of motion.
Root Mean Square (RMS) Speed: A measure of the average molecular speed, often explained by the Boltzmann distribution.
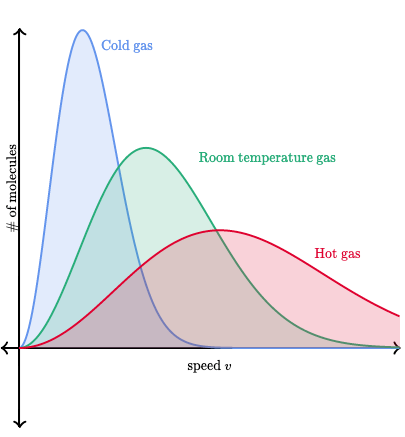
Boltzmann Distribution Insight:
Higher temperatures increase molecular speed.
The peak of the distribution decreases as the spread widens with temperature.
Temperature and Heat: What’s the Difference?
Temperature: Measures an object’s internal energy. Directly related to average kinetic energy via:
Heat: Measures energy transfer between objects due to temperature differences. Heat flows from hot to cold objects until thermal equilibrium is reached.
Thermal Equilibrium:
When two objects have the same temperature and no net heat transfer occurs.
Molecular collisions and energy exchanges still happen, but the overall energy remains constant.
Key Differences:
| Heat | Temperature |
|---|---|
| Energy transferred between objects. | Measures average kinetic energy. |
| Units: Joules (J), Calories (cal). | Units: Degrees Celsius, Fahrenheit. |
| Describes interaction. | Intrinsic property of an object. |
The Ideal Gas Law
The Ideal Gas Law is fundamental in science and engineering. It combines multiple gas laws into one:
Where:
: Pressure (Pa or atm)
: Volume (m³ or L)
: Number of moles
: Universal gas constant (8.314 J/(mol·K))
: Temperature (K)
Underlying Laws:
Charles’ Law: (Volume increases with temperature.)
Gay-Lussac’s Law: (Pressure increases with temperature.)
Avogadro’s Law: (Volume increases with more moles.)
Boyle’s Law: (Pressure decreases as volume increases.)
The Ideal Gas Law encapsulates these relationships, offering a comprehensive description of gas behavior.
Assumptions of the Ideal Gas Law:
Molecules occupy no volume: Real molecules do, but this is ignored.
No intermolecular forces: Neglects attractive/repulsive interactions.
Elastic Collisions: Molecules don’t lose kinetic energy upon collision.
Applicability:
Works best at high temperatures and low pressures where gases behave ideally.
For small quantities, the Ideal Gas Law can be rewritten using the Boltzmann constant:
Where is the number of molecules.
Limitations:
Real gases deviate from ideal behavior under extreme conditions. The Van der Waals equation accounts for these deviations but is not required for the AP exam.
Key Takeaways:
The Ideal Gas Law is a cornerstone for predicting gas behavior under varying conditions.
Understand its limitations and assumptions for accurate application in real-world scenarios.
By mastering these concepts, you’ll gain a deeper understanding of how pressure, temperature, and molecular motion interconnect in thermodynamics. Perfecting this knowledge will help you solve practical problems and ace related questions on the AP exam! 🌟







