4.1 Open and Closed Systems: Energy
Conservation Laws 👨💻
Energy is a fundamental quantity in the universe, governed by the law of conservation of energy, which states:
Energy cannot be created or destroyed, only transformed from one form to another.
This principle ensures that the total energy in a closed system remains constant. For example:
- Energy lost by one part of a system is gained by another.
- Applies to kinetic energy (motion), potential energy (position/configuration), and thermal energy (heat).
Total Energy Changes 👨💻
Interactions with other systems or objects can alter the total energy of a system. Understanding energy transfer requires clear distinctions between systems and objects.
Systems vs. Objects 💡
What is a System?
A system is a defined group of objects being studied, treated as if they have no internal structure. Examples:
- A ball and Earth (gravitational interaction).
- A car and the road (mechanical interaction).
Defining a system helps differentiate:
- Internal Forces: Forces caused by members within the system.
- External Forces: Forces caused by objects outside the system.
Types of Systems
Open Systems:
- Exchange matter and energy with surroundings.
- Example: A pot of boiling water releases steam and heat.
Closed Systems:
- Exchange energy, but not matter, with surroundings.
- Example: A sealed soda can transfers heat but retains its contents.
Isolated Systems:
- Exchange neither matter nor energy.
- Example: A perfectly insulated thermos (idealized).
Force Interactions 💡
Interactions occur via:
- Forces exerted by objects outside the system.
- Transfer of quantities like energy or momentum with external objects.
When analyzing problems involving energy and work, identifying the system simplifies calculations.
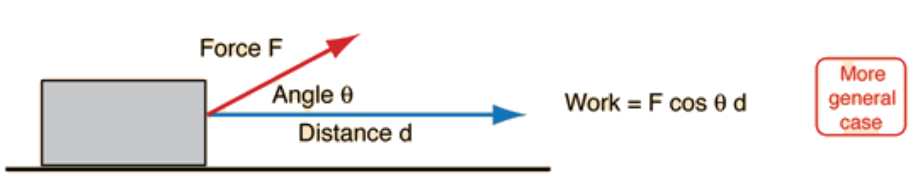
Work: Transfer of Energy
Definition
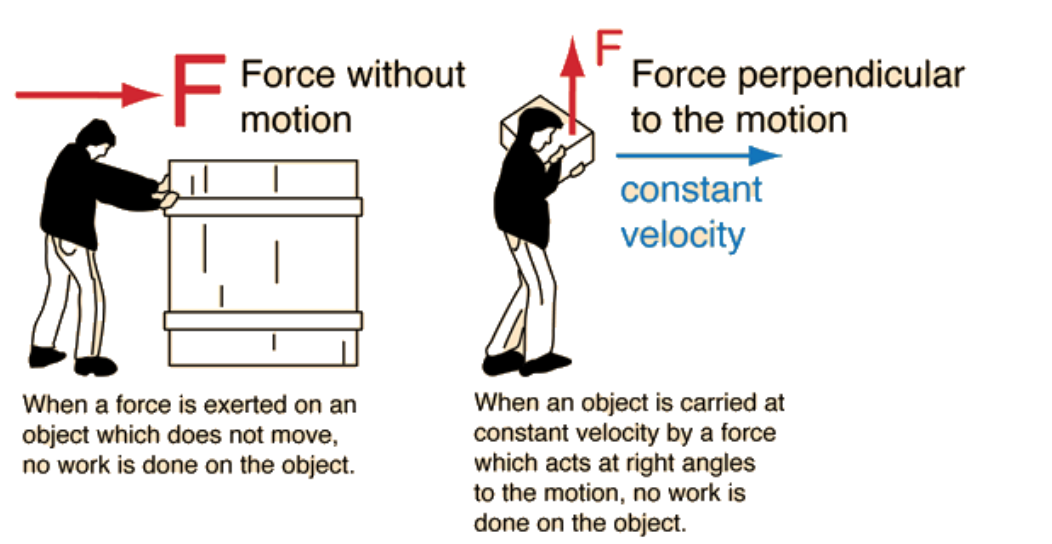
Work occurs when a force causes an object to move in the direction of the force.
- : Work.
- : Force applied.
- : Distance moved.
- : Angle between the force and displacement vectors.
Key Points
- Positive work adds energy to the system.
- Negative work removes energy from the system.
- Work can also be calculated graphically from a Force vs. Displacement graph, using geometric shapes like rectangles and triangles to find the area under the curve.
Power
Power measures the rate at which work is done or energy is transferred:
- : Power.
- : Energy change (Work).
- : Time.
Key Points About Power
- Measured in watts (W) or joules per second (J/s).
- Scalar quantity (has only magnitude, no direction).
- Can be positive or negative depending on the direction of energy transfer.
- Indicates how fast energy is transferred or a task is completed.
Summary
- Energy Conservation: Total energy in a closed system remains constant, though it can transfer or transform.
- Systems and Forces: Clearly define the system to differentiate internal and external forces.
- Work: Transfers energy, with positive work increasing and negative work decreasing system energy.
- Power: Measures the rate of energy transfer or work done, offering insights into efficiency and performance.
Real-World Examples
- Boiling Water (Open System): Energy from the stove heats water, which releases steam into the air.
- Sealed Soda Can (Closed System): Heat can transfer in or out, but no matter escapes.
- Roller Coaster: Transfers gravitational potential energy to kinetic energy, with work done by external forces like friction affecting total energy.







